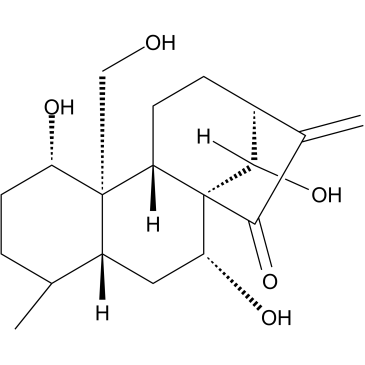
Kamebakaurin |
| Catalog No.GC39093 |
Kamebakaurin, a compound of kaurane diterpenes was isolated from traditional Chinese medicinal plant Isodon excia.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 73981-34-7
Sample solution is provided at 25 µL, 10mM.
Kamebakaurin, a compound of kaurane diterpenes was isolated from traditional Chinese medicinal plant Isodon excia. It is a potent inhibitor of NF-kappaB activation by directly targeting DNA-binding activity of p50[6].
Treated with different concentrations of kamebakaurin (0-30µM;24 h), The activity of HCT116 cells did not decrease significantly, but Kamebakaurin inhibits HIF-1α protein expression in cells [1]. Kamebakaurin (0.1, 1.0, 5 µM) significantly inhibited the LPS-induced production of nitric oxide (NO) in a concentration-dependent fashion in activated microglial cells[2]. Kamebakaurin (0-500ng/ml;4h)dose-dependently attenuated iNOS gene expression in LPS-activated dendritic cells (DCs). Kamebakaurin significantly inhibited the gene expression and protein production of the inflammatory cytokines TNF-α, IL-12, and IL-1β[4].
Kamebakaurin (50 mg/kg;40 days;p.o.) produced significant growth inhibition of HCT116 cells in tumor xenograft model[1]. Kamebakaurin dose-dependently suppressed the inflammation in an adjuvant arthritis model. Oral administration of 20 mg/kg kamebakaurin resulted in the 75% decrease of paw volume[3]. Pretreatment with Kamebakaurin reduced the magnitude of Acetaminophen (N-acetyl-p-aminophenol, APAP)-induced increases in plasma levels of hepatic injury markers, lipid peroxidation, and inflammatory response[5].
References:
[1]. Wang KS, Ma J, et,al. Kamebakaurin inhibits the expression of hypoxia-inducible factor-1α and its target genes to confer antitumor activity. Oncol Rep. 2016 Apr;35(4):2045-52. doi: 10.3892/or.2016.4576. Epub 2016 Jan 19. PMID: 26781327.
[2]. Kim BW, Koppula S, et,al.Anti-neuroinflammatory activity of Kamebakaurin from Isodon japonicus via inhibition of c-Jun NH?-terminal kinase and p38 mitogen-activated protein kinase pathway in activated microglial cells. J Pharmacol Sci. 2011;116(3):296-308. doi: 10.1254/jphs.10324fp. Epub 2011 Jun 25. PMID: 21705843.
[3]. Lee JH, Choi JK, et,al. Anti-inflammatory effect of kamebakaurin in in vivo animal models. Planta Med. 2004 Jun;70(6):526-30. doi: 10.1055/s-2004-827152. PMID: 15241890.
[4]. Kim JY, Kim HS, et,al. Inhibition of TAK1 by kamebakaurin in dendritic cells. Int Immunopharmacol. 2013 Jan;15(1):138-43. doi: 10.1016/j.intimp.2012.11.004. Epub 2012 Nov 15. PMID: 23159603.
[5]. Yoshioka H, Aoyagi Y, et,al. Suppressive effect of kamebakaurin on acetaminophen-induced hepatotoxicity by inhibiting lipid peroxidation and inflammatory response in mice. Pharmacol Rep. 2017 Oct;69(5):903-907. doi: 10.1016/j.pharep.2017.04.004. Epub 2017 Apr 12. PMID: 28624597.
[6]. Lee JH, et,al.Kaurane diterpene, kamebakaurin, inhibits NF-kappa B by directly targeting the DNA-binding activity of p50 and blocks the expression of antiapoptotic NF-kappa B target genes. J Biol Chem. 2002 May 24;277(21):18411-20. doi: 10.1074/jbc.M201368200. Epub 2002 Mar 4. PMID: 11877450.
Average Rating: 5 (Based on Reviews and 26 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















