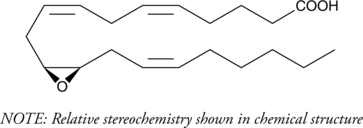
(±)11(12)-EET (Synonyms: (±)11,12-EpETrE) |
| Catalog No.GC40466 |
(±)11(12)-EET는 NLRP3 인플라마좀 억제제입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 123931-40-8
Sample solution is provided at 25 µL, 10mM.
(±)11(12)-EET is a fully racemic version of the R/S enantiomeric forms biosynthesized from arachidonic acid by cytochrome P450 enzymes.[1][2][3[] A higher proportion of 11(R),12(S)-EET is produced by the CYP450 isoforms CYP2C23 and CYP2C24 while CYP2B2 produces a higher proportion of 11(S),12(R)-EET.[3] 11(12)-EET has been shown, along with 8(9)-EET to play a role in the recovery of depleted calcium pools in cultured smooth muscle cells[4] It also inhibits basolateral 18-pS potassium channels in the renal cortical collecting duct when used at a concentration of 100 nM.[5] 11(12)-EET (50 µg/kg per day) increases adhesion of isolated peripheral blood leukocytes in a chamber coated with P-selectin and ICAM-1 but does not affect choroidal neovascularization size following laser photocoagulation[6] It also has anti-inflammatory, angiogenic, and cardioprotective properties[7]
Reference:
[1]. Chacos, N., Falck, J.R., Wixtrom, C., et al. Novel epoxides formed during the liver cytochrome P-450 oxidation of arachidonic acid. Biochem. Biophys. Res. Commun. 104(3), 916-922 (1982).
[2]. Oliw, E.H., Guengerich, F.P., and Oates, J.A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J. Biol. Chem. 257(7), 3771-3781 (1982).
[3]. Capdevila, J.H., Falck, J.R., and Harris, R.C. Cytochrome P450 and arachidonic acid bioactivation: Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 41(2), 163-181 (2000).
[4]. Graber, M.N., Alfonso, A., and Gill, D.L. Recovery of Ca2+ pools and growth in Ca2+ pool-depleted cells is mediated by specific epoxyeicosatrienoic acids derived from arachidonic acid. J. Biol. Chem. 272(47), 29546-29553 (1997).
[5]. Wang, Z., Wei, Y., Falck, J.R., et al. Arachidonic acid inhibits basolateral K channels in the cortical collecting duct via cytochrome P-450 epoxygenase-dependent metabolic pathways. Am. J. Physiol. Renal Physiol. 294(6), F1441-F1447 (2008).
[6]. Hasegawa, E., Inafuku, S., Mulki, L., et al. Cytochrome P450 monooxygenase lipid metabolites are significant second messengers in the resolution of choroidal neovascularization. Proc. Natl. Acad. Sci. U.S.A. 114(36), E7545-E7553 (2017).
[7]. Spector, A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 50(Suppl), S52-S56 (2009).
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















