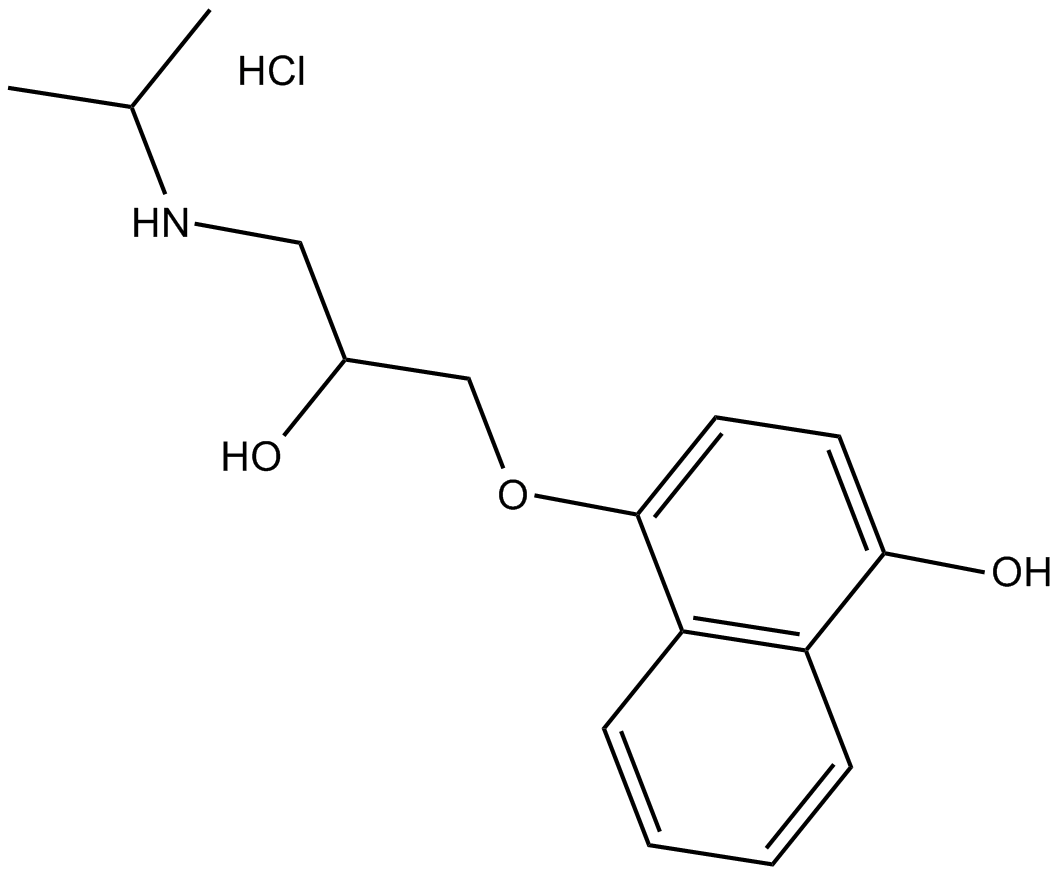
(±)-4-hydroxy Propranolol (hydrochloride) |
| Catalog No.GC16663 |
4-Hydroxypropranolol 염산염은 Propranolol의 활성 대사 산물입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 14133-90-5
Sample solution is provided at 25 µL, 10mM.
IC50: 1.1 μM: inhibits lipid peroxidation in endothelial cells.
(±)-4-hydroxy Propranolol, an active metabolite of propranolol, blocks β1- and β2-adrenergic receptors (β1-ARs, β2-ARs). Also, (±)-4-hydroxy propranolol has antioxidant properties at micromolar concentrations. β1- and β2-ARs, expressed in cardiac myocytes, mediate an increase in contractility by Gs-dependent coupling to adenylyl cyclase.
In vitro: Compared to the control, (±)-4-hydroxy propranolol potently blocked the lipid peroxidation in a concentration-dependent fashion in endothelial cells. When pretreated with (±)-4-hydroxy propranolol at 0.067 to 6.7 μM, the degrees of protection were increased against the glutathione loss in the endothelial cells. Additionally, (±)-4-hydroxy propranolol effectively preserved the loss of cell survival because of the radical stress [1].
In vivo: Rats were injected intravenously with (±)-4-hydroxy propranolol into the femoral vein at 0.1 ml/100 g. (±)-4-hydroxy Propranolol induced an increase in heart rate in a dose-dependent manner in rats depleted of catecholamines, which suggested that (±)-4-hydroxy propranolol had intrinsic sympathomimetic activity. The response of (±)-4-hydroxy propranolol was inhibited when rats were pretreated with 0.5 mg/kg propranolol [2].
References:
[1]. Mak, I. Potent Antioxidant Properties of 4-Hydroxyl-propranolol. Journal of Pharmacology and Experimental Therapeutics. 2003; 308(1): 85-90.
[2]. FITZGERALD, J., & O'DONNELL, S. Pharmacology of 4-hydroxypropranolol, a metabolite of propranolol. British Journal of Pharmacology. 1971; 43(1): 222-235.
Average Rating: 5 (Based on Reviews and 2 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















