(±)-Huperzine A (Synonyms: Hup A, (-)-Selagine) |
| Catalog No.GC11965 |
A neuroprotective AChE inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
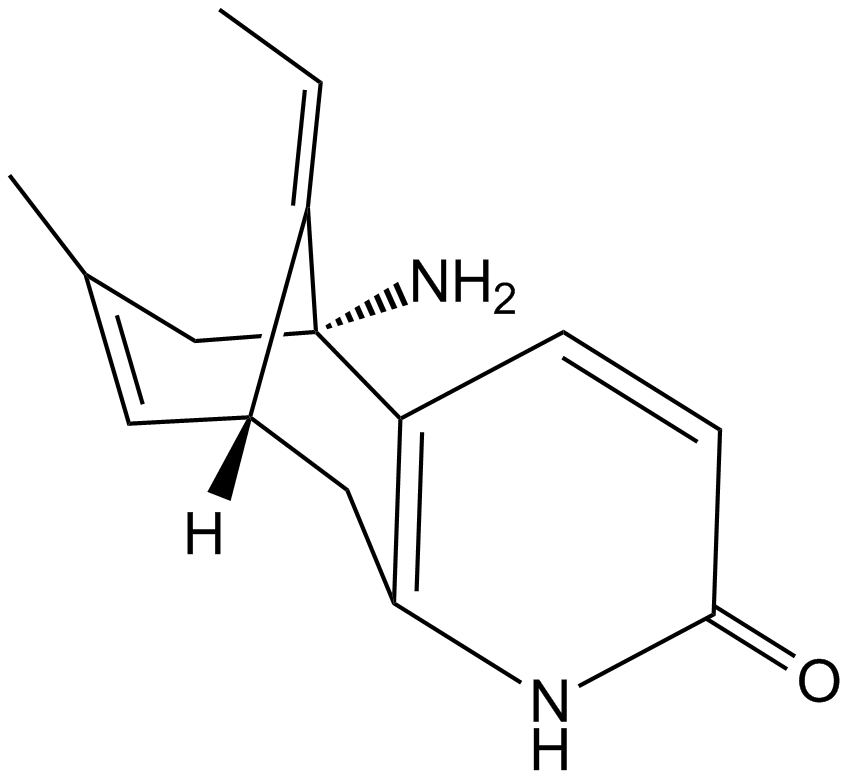
Cas No.: 120786-18-7
Sample solution is provided at 25 µL, 10mM.
(−)-Huperzine A (HupA) is an acetylcholinesterase (AChE) inhibitor with an IC50 value of 82 nmol/L [1] and acts as an antagonist of the N-methyl-d-aspartate (NMDA) receptor [2].
AChE is the key brain enzyme responsible for the rapid degradation of the neurotransmitter acetylcholine. AChE inhibitors are probably useful in the amelioration of the Alzheimer’s symptomatology [3].
It was found that NMDA markedly reduced AChE activities [4]. In rat dissociated hippocampal neurons, HupA inhibited the NMDA-induced current. In neurons, 100 µM HupA, NMDA-induced currents were 55.7 ± 4.9% of the control values. The binding molecular ratio of NMDA receptor: HupA is 1:1. The inhibition of NMDA receptor by HupA is not competitive [5]. HupA significantly increased the phosphorylation levels of both glycogen synthase kinase (GSK)-3α protein and GSK-3β protein in APPsw-overexpressing cells [2]. Activated GSK-3 consequently decreased acetylcholine (ACh) level in the striatum [6].
Treated with doses of (−)-huperzine A, AChE−/− mice showed no toxic symptoms and had normal levels of AChE. This demonstrated the specificity of (−)-huperzine A as an inhibitor of AChE at the dose used in vivo [7]. In rat whole brain, oral administration of HupA at a dose of 1.5 μmol/kg (3.6 mg/kg) obtained a maximum inhibition of AChE at 60 min and this maximum inhibition was maintained for 360 min [8].
References:
[1]. MA Xiao-Chao, XIN Jian, WANG Hai-Xue, et al. Acute effects of huperzine A and tacrine on rat liver. Acta Pharmacol ogica Sinica, 2003, 24(3):247-250.
[2]. Zhong Ming Qian and Ya Ke. Huperzine A: is it an effective disease-modifying drug for Alzheimer's disease? Frontiers in Aging Neuroscience, 2014, 6:216.
[3]. V. Rajendran, Suo-Bao Rong, Ashima Saxena, et al. Synthesis of a hybrid analog of the acetylcholinesterase inhibitors huperzine A and huperzine B. Tetrahedron Letters, 2001, 42: 5359-5361.
[4]. J. R. Delfs, D. M. Saroff, Y. Nishida, et al. Effects of NMDA and its antagonists on ventral horn cholinergic neurons in organotypic roller tube spinal cord cultures. J. Neural Transm., 1997, 104(1):31-51.
[5]. J. M. Zhang and G. Y. Hu. Huperzine A, a nootropic alkaloid, inhibits N-methyl-D-aspartate-induced current in rat dissociated hippocampal neurons. Neuroscience, 2001, 105(3):663-9.
[6]. L. Zhao, C. B. Chu, J. F. Li, et al. Glycogen synthase kinase-3 reduces acetylcholine level in striatum via disturbing cellular distribution of choline acetyltransferase in cholinergic interneurons in rats. Neuroscience, 2013, 255:203-11.
[7]. Ellen G. Duysen, Bin Li, Sultan Darvesh, et al. Sensitivity of butyrylcholinesterase knockout mice to (−)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer’s disease drugs and indicates butyrylcholinesterase function in neurotransmission. Toxicology, 2007, 233:60-69.
[8]. Rui Wang, Han Yan and Xi-can Tang. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacologica Sinica, 2006, 27:1-26.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















