Proteases
Proteases is a general term for a class of enzymes that hydrolyze protein peptide chains. According to the way they degrade polypeptides, they are divided into two categories: endopeptidases and telopeptidases. The former can cut the large molecular weight polypeptide chain from the middle to form prions and peptones with smaller molecular weights; the latter can be divided into carboxypeptidase and aminopeptidase, which respectively remove the peptide from the free carboxyl terminus or free amino terminus of the polypeptide one by one. Chain hydrolysis produces amino acids.
A general term for a class of enzymes that hydrolyze peptide bonds in proteins. According to the way they hydrolyze polypeptides, they can be divided into endopeptidases and exopeptidases. Endopeptidase cleaves the interior of the protein molecule to form smaller molecular weight peptones and peptones. Exopeptidase hydrolyzes peptide bonds one by one from the end of the free amino group or carboxyl group of protein molecules, and frees amino acids, the former is aminopeptidase and the latter is carboxypeptidase. Proteases can be classified into serine proteases, sulfhydryl proteases, metalloproteases and aspartic proteases according to their active centers and optimum pH. According to the optimum pH value of its reaction, it is divided into acidic protease, neutral protease and alkaline protease. The proteases used in industrial production are mainly endopeptidases.
Proteases are widely found in animal offal, plant stems and leaves, fruits and microorganisms. Microbial proteases are mainly produced by molds and bacteria, followed by yeast and actinomycetes.
Enzymes that catalyze the hydrolysis of proteins. There are many kinds, the important ones are pepsin, trypsin, cathepsin, papain and subtilisin. Proteases have strict selectivity for the reaction substrates they act on. A protease can only act on certain peptide bonds in protein molecules, such as the peptide bonds formed by the hydrolysis of basic amino acids catalyzed by trypsin. Proteases are widely distributed, mainly in the digestive tract of humans and animals, and are abundant in plants and microorganisms. Due to limited animal and plant resources, the industrial production of protease preparations is mainly prepared by fermentation of microorganisms such as Bacillus subtilis and Aspergillus terrestris.
Targets for Proteases
- Caspase(114)
- Aminopeptidase(24)
- ACE(74)
- Calpains(20)
- Carboxypeptidase(10)
- Cathepsin(81)
- DPP-4(31)
- Elastase(26)
- Gamma Secretase(67)
- HCV Protease(59)
- HSP(113)
- HIV Integrase(37)
- HIV Protease(47)
- MMP(228)
- NS3/4a protease(8)
- Serine Protease(18)
- Thrombin(58)
- Urokinase(4)
- Cysteine Protease(0)
- Other Proteases(18)
- Tyrosinases(47)
- 15-PGDH(1)
- Acetyl-CoA Carboxylase(13)
- Acyltransferase(59)
- Aldehyde Dehydrogenase (ALDH)(28)
- Aminoacyl-tRNA Synthetase(9)
- ATGL(1)
- Dipeptidyl Peptidase(56)
- Drug Metabolite(457)
- E1/E2/E3 Enzyme(90)
- Endogenous Metabolite(1636)
- FABP(30)
- Farnesyl Transferase(23)
- Glutaminase(14)
- Glutathione Peroxidase(14)
- Isocitrate Dehydrogenase (IDH)(28)
- Lactate Dehydrogenase(17)
- Lipoxygenase(234)
- Mitochondrial Metabolism(207)
- NEDD8-activating Enzyme(7)
- Neprilysin(12)
- PAI-1(13)
- Ser/Thr Protease(41)
- Tryptophan Hydroxylase(13)
- Xanthine Oxidase(18)
- MALT1(10)
- PCSK9(1)
Products for Proteases
- Cat.No. 상품명 정보
-
GC49118
10-hydroxy Warfarin
A metabolite of (R)-warfarin
-
GC33800
10Z-Nonadecenoic acid
10Z-노나데센산은 항종양 활성이 있는 일종의 장쇄 지방산입니다.
-
GC40368
11(R)-HEPE
11(R)-HEPE is produced by the oxidation of EPA by 11(R)-LO.
-
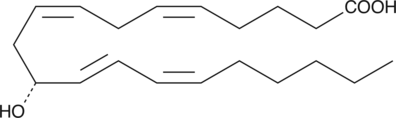
GC40445
11(R)-HETE
11(R)-HETE is biosynthesized by 11(R)-LOs of the sea urchin, S.
-
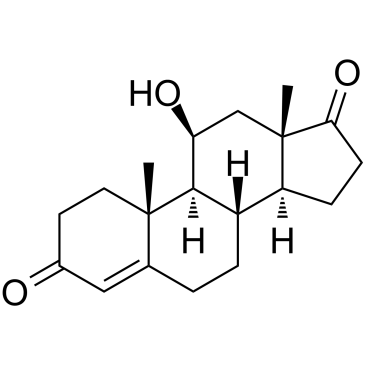
GC39223
11-Beta-hydroxyandrostenedione
11-베타-하이드록시안드로스텐디온(4-안드로스텐-11β-올-3,17-디온)은 주로 부신 기원에서 발견되는 스테로이드입니다(11β-하이드록실라제는 부신 조직에 존재하지만 난소 조직에는 존재하지 않음).
-
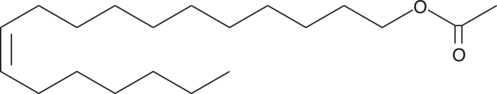
GC40730
11-cis Vaccenyl Acetate
11-cis Vaccenyl Acetate는 수컷과 암컷 파리 모두에서 응집 행동을 매개하는 수컷 특이적 지질로, 수컷과 암컷 파리 모두의 안테나에 있는 T1 센실라에 위치한 수십 개의 후각 뉴런을 활성화합니다.
-
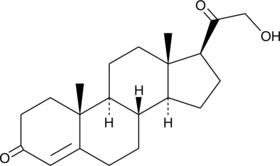
GC40394
11-deoxy Corticosterone
11-디옥시 코르티코스테론은 부신에서 생성되는 스테로이드 호르몬으로, 미네랄코르티코이드 활성을 가지며 알도스테론 전구체로 작용합니다.
-
GC10821
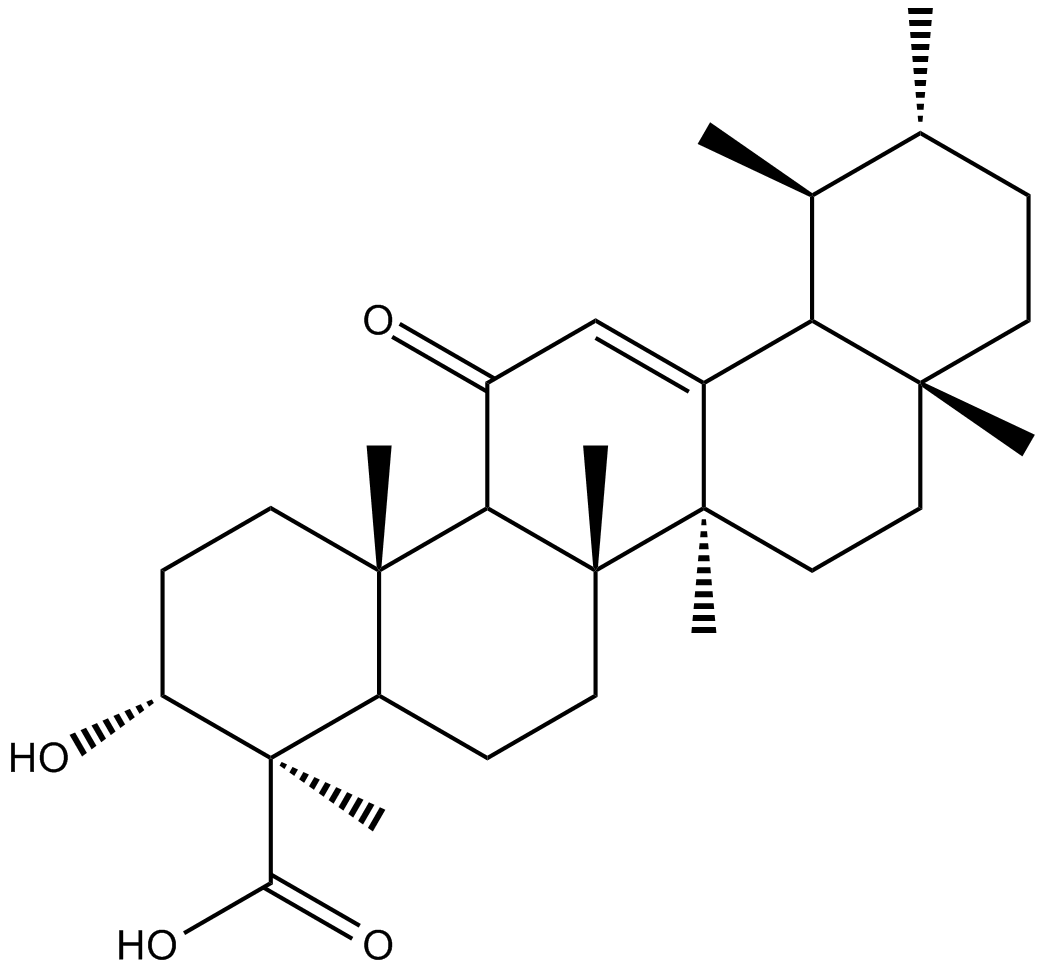
11-keto-β-Boswellic Acid
11-Keto-beta-boswellic acid(11-Keto-β-boswellic acid)는 인도 유향으로 널리 알려진 Boswellia serrate tree의 껍질에서 추출한 올레오검 수지의 5환형 트리테르펜산입니다. 11-Keto-beta-boswellic acid는 주로 5-lipoxygenase(5-LOX) 및 후속 류코트리엔 및 핵 인자-카파 B(NF-κB) 활성화 및 종양 괴사 인자 알파 생성을 억제하기 때문에 항염증 활성이 있습니다. 생산.
-
GC61538
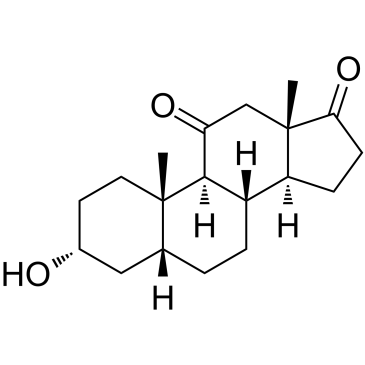
11-Oxo etiocholanolone
11-Oxo etiocholanolone (11-Ketoetiocholanolone)은 Etiocholanolone의 대사 산물입니다.
-
GC41144
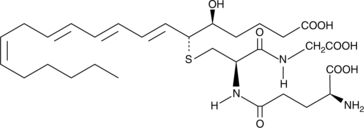
11-trans Leukotriene C4
11-trans Leukotriene C4 (11-trans LTC4) is a C-11 double bond isomer of LTC4.
-
GC41147
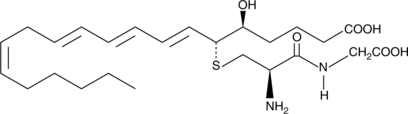
11-trans Leukotriene D4
11-trans Leukotriene D4 (11-trans LTD4) is a C-11 double bond isomer of LTD4.
-
GC41149
11-trans Leukotriene E4
11-trans 류코트리엔 E4는 류코트리엔 E4(LTE4)의 이성질체입니다.
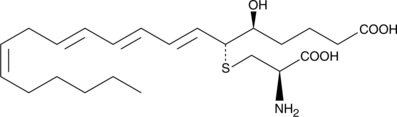
-
GC63796
116-9e
116-9e(MAL2-11B)는 Hsp70 공동 샤페론 DNAJA1 억제제입니다.
-
GC34016
11beta-Hydroxyprogesterone (11β-Hydroxyprogesterone)
11베타-하이드록시프로게스테론(11&베타;-하이드록시프로게스테론)은 11β의 강력한 억제제입니다. 또한 ED50이 10nM인 COS-7 세포에서 인간 미네랄 코르티코이드 수용체를 활성화합니다.
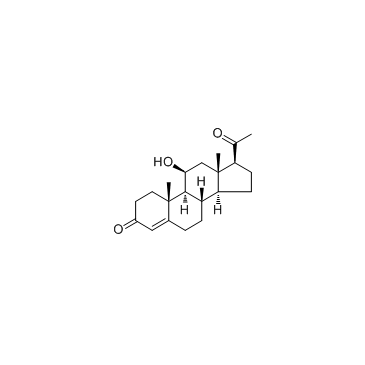
-
GC40447
12(R)-HETE
Biosynthesis of 12(R)-HETE in invertebrates is via lipoxygenation of arachidonic acid.
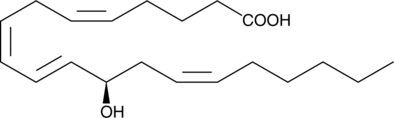
-
GC40371
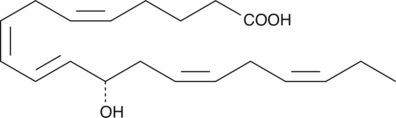
12(S)-HEPE
12(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 12-LO.
-
GC40448
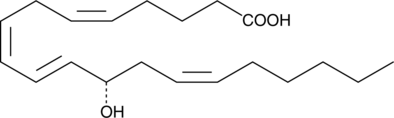
12(S)-HETE
12(S)-HETE is the predominant lipoxygenase product of mammalian platelets.
-
GC41882
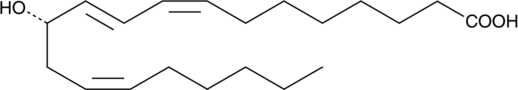
12(S)-HETrE
12(S)-HETrE is produced by 12-lipoxygenase oxidation of dihomo-γ-linolenic acid (DGLA).
-
GC41095
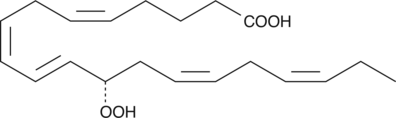
12(S)-HpEPE
12(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 12-lipoxygenase on eicosapentaenoic acid.
-
GC41122
12(S)-HpETE
12(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of platelet or leukocyte 12-lipoxygenase (12-LO) on arachidonic acid.
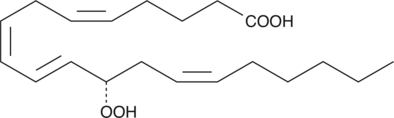
-
GC41123
12-epi Leukotriene B4
Leukotriene B4 (LTB4) compounds are produced by both enzymatic and non-enzymatic processes.
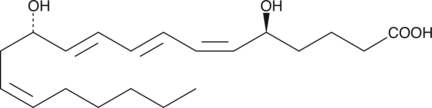
-
GC45962
12-hydroxy Lauric Acid
12-하이드록시라우르산은 내인성 대사산물입니다.
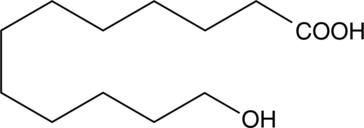
-
GC60443
12-Ketodeoxycholic acid
12-케토데옥시콜산은 담즙산으로 신장에서 대사산물입니다.
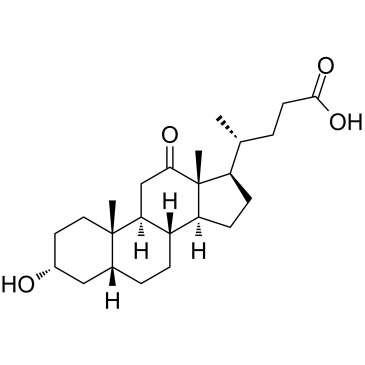
-
GC41096
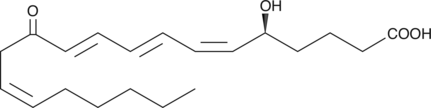
12-oxo Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-LO pathway.
-
GC46418
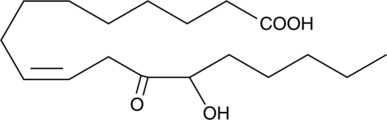
12-oxo-13-HOME
An oxylipin
-
GC40372
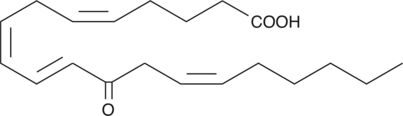
12-OxoETE
12-OxoETE is synthesized by human platelets and Aplysia nervous tissue after incubation with arachidonic acid.
-
GC19462
13(R)-HODE
13(R)-HODE is the opposite enantiomer of the 13(S)-HODE produced when linoleic acid is incubated with soybean lipoxygenase.

-
GC19463
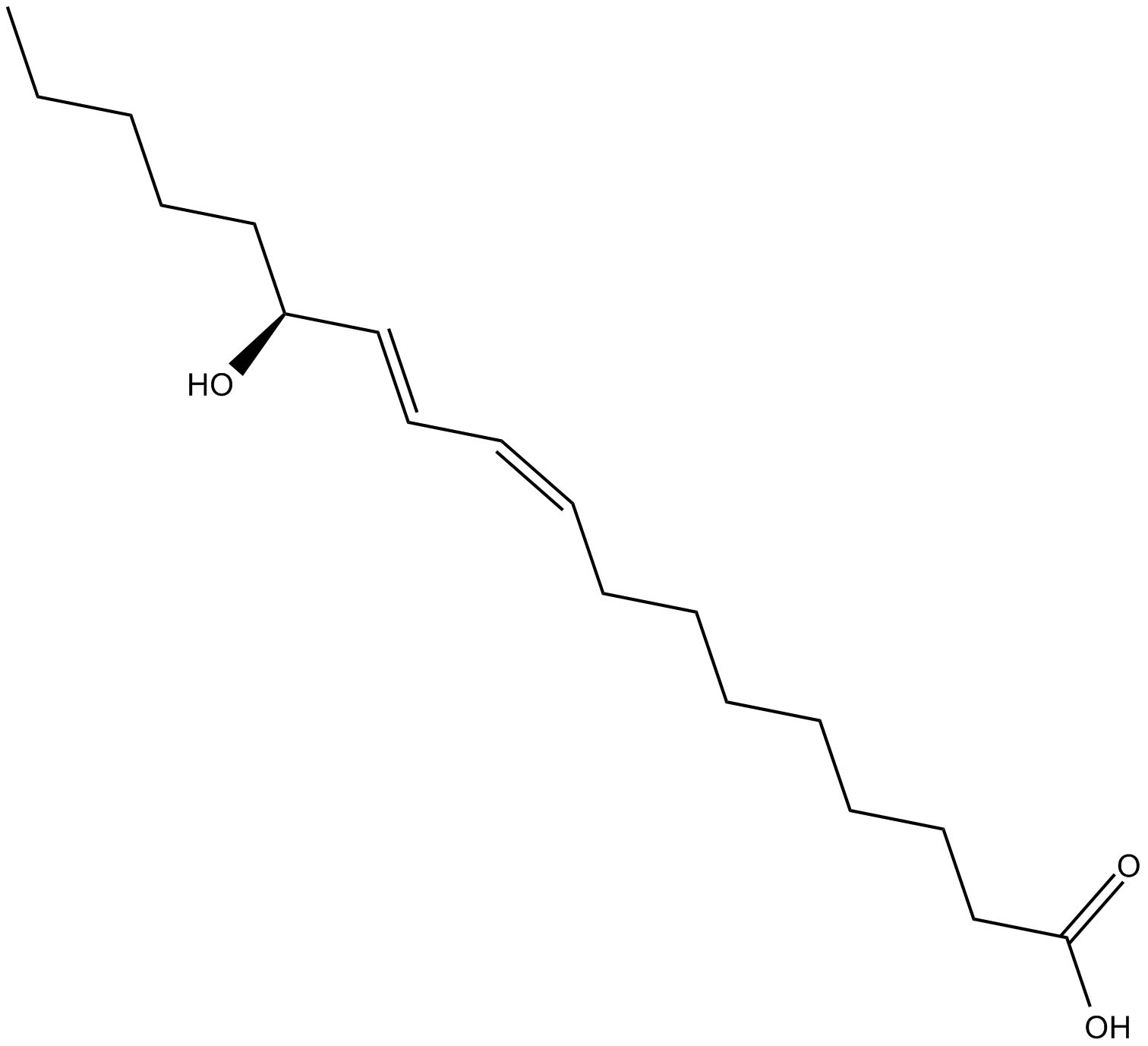
13(S)-HODE
리놀레산의 15-리폭시게나제(15-LOX) 대사 산물인 13(S)-HODE(13(S)-HODE)는 PPARγ을 활성화하는 내인성 리간드로 기능합니다.
-
GC41220
13(S)-HODE methyl ester
13(S)-hydroxyoctadecadienoic acid (13(S)-HODE) is a 15-lipoxygenase metabolite of linoleic acid produced in endothelial cells, leukocytes, and tumor cells.
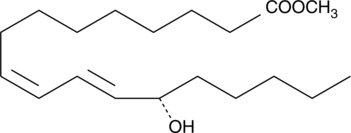
-
GC41896
13(S)-HODE-biotin
13(S)-HODE is the lipoxygenase metabolite of linoleic acid.
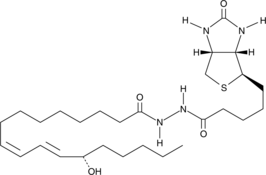
-
GC46420
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE
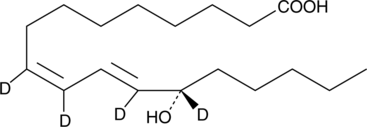
-
GC41897
13(S)-HOTrE
13(S)-HOTrE is the 15-lipoxygenase (15-LO) product of linolenic acid.
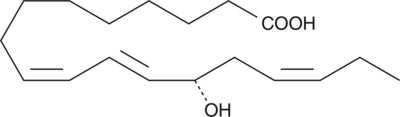
-
GC41898
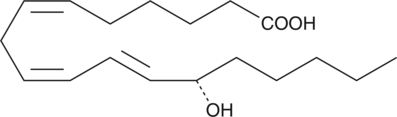
13(S)-HOTrE(γ)
13(S)-HOTrE(γ) is the 15-LO product of γ-linolenic acid.
-
GC19474
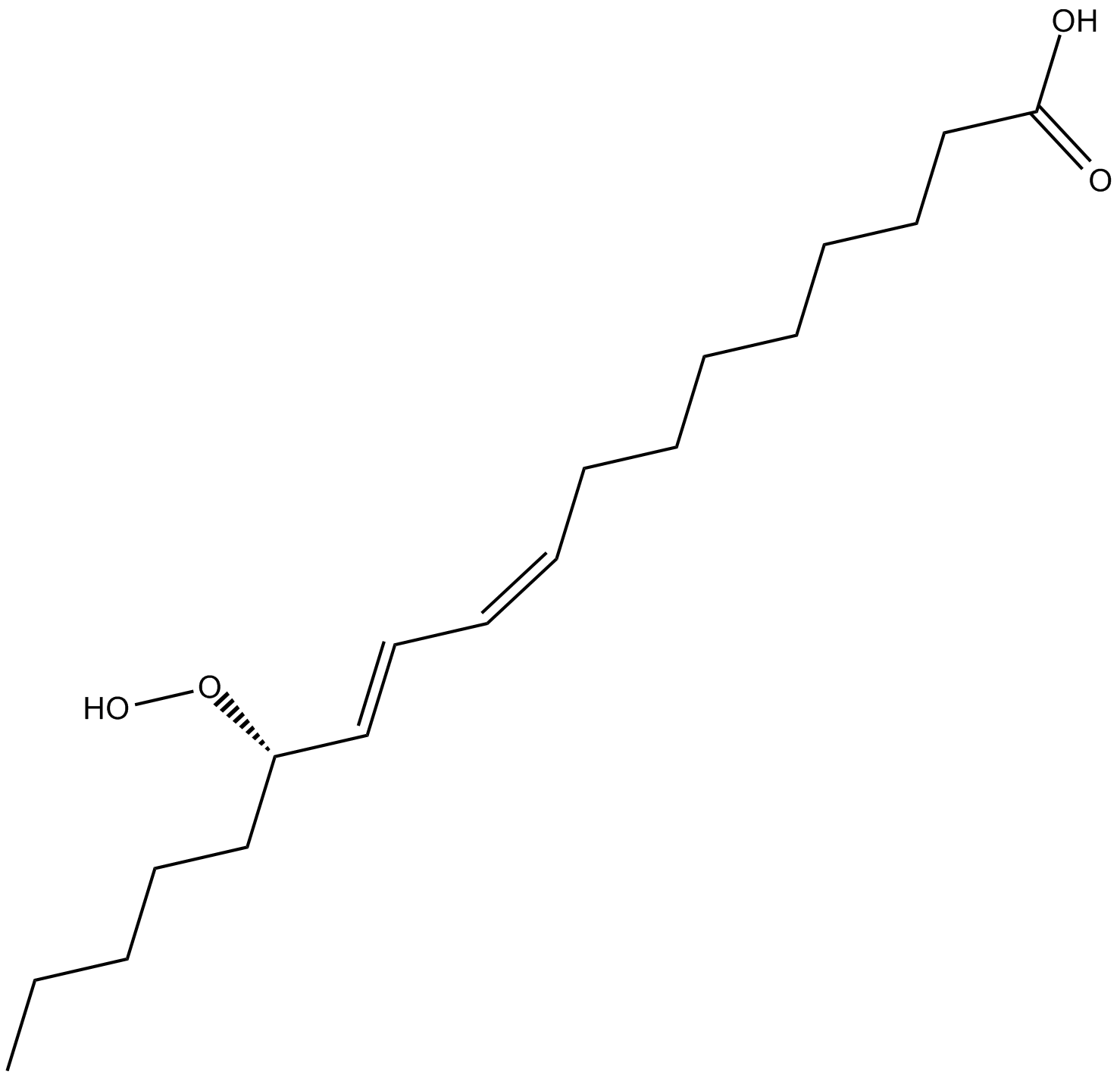
13(S)-HpODE
13(S)-HpODE is produced by the oxidation of linoleic acid by lipoxygenase-1 (LO-1) in many plants including soybean, flaxseed, apples, and tea leaves,1,2 and by 15-LO in mammals.
-
GC41899
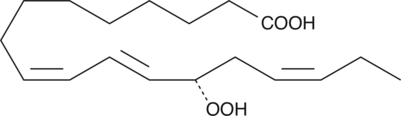
13(S)-HpOTrE
13(S)-HpOTrE is a monohydroperoxy polyunsaturated fatty acid produced in soybeans by the action of soybean LO-2 on esterified α-linolenic acid.
-
GC41900
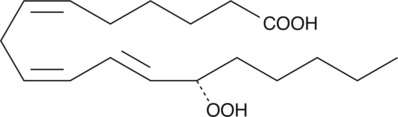
13(S)-HpOTrE(γ)
13(S)-HpOTrE(γ) is a monohydroxy PUFA produced by the action of soybean lipoxygenase-1 (LO-1) on γ-linolenic acid.
-
GC62758
13-cis-Vitamin A palmitate
13-시스-비타민 A 팔미테이트(13-시스-레티닐 팔미테이트)는 콘플레이크의 비타민 A 팔미테이트에 의해 형성된 13-시스 이성질체입니다.
-
GC41911
13-epi-12-oxo Phytodienoic Acid
13-epi-12-oxo Phytodienoic acid (13-epi-12-oxo PDA) is a lipoxygenase metabolite of α-linolenic acid in the leaves of green plants such as corn.
-
GC49759
13C17-Mycophenolic Acid
An internal standard for the quantification of mycophenolic acid
-
GC41206
14(S)-HDHA
14(S)-HDHA(14(S)-HDoHE)는 도코사헥사엔산(DHA)의 산소화 생성물입니다.
-
GC41100
14,15-dehydro Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-lipoxygenase pathway.
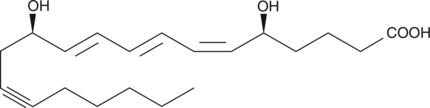
-
GC41145
14,15-Leukotriene C4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
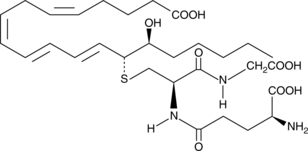
-
GC41148
14,15-Leukotriene D4
14,15-Leukotriene D4 (14,15-LTD4) is a member of an alternate class of LTs synthesized by a pathway involving the dual actions of 15- and 12-lipoxygenases (15- and 12-LOs) on arachidonic acid via 15-HpETE and 14,15-LTA4 intermediates.
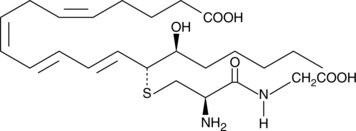
-
GC41150
14,15-Leukotriene E4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
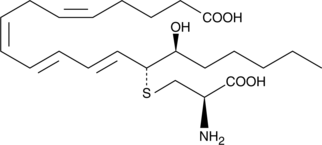
-
GC41415
15(R)-Lipoxin A4
Lipid-derived lipoxins are produced at the site of vascular and mucosal inflammation where they down-regulate polymorphonuclear leukocyte recruitment and function.
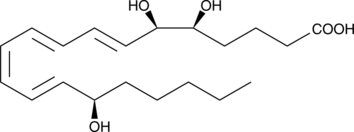
-
GC40427
15(S)-HEDE
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO.
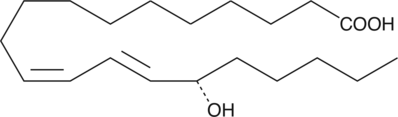
-
GC40373
15(S)-HEPE
15(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 15-LO.
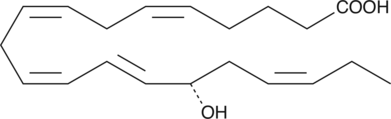
-
GC40451
15(S)-HETE
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
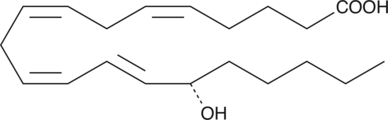
-
GC41925
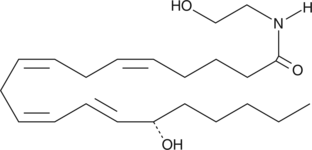
15(S)-HETE Ethanolamide
Arachidonoyl ethanolamide was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.
-
GC40839
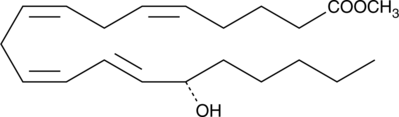
15(S)-HETE methyl ester
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE, a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
-
GC46442
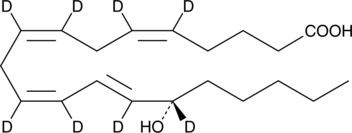
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
-
GC49894
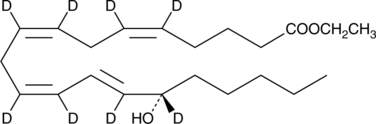
15(S)-HETE-d8 ethyl ester
An internal standard for the quantification of 15(S)-HETE ethyl ester
-
GC41927
15(S)-HETrE
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid.
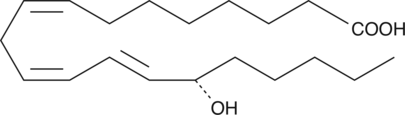
-
GC41403
15(S)-HpEDE
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid.
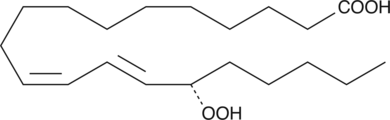
-
GC41101
15(S)-HpEPE
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid.
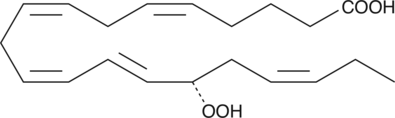
-
GC41124
15(S)-HpETE
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid.
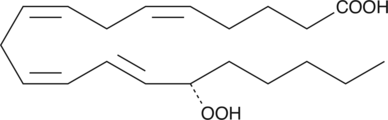
-
GC11988
15-acetoxy Scirpenol
ASM(acetoxyscirpenol moiety mycotoxins) 중 하나인 15-acetoxyscirpenol은 caspase-3와 무관한 다른 caspase를 활성화하여 용량 의존적으로 Jurkat T 세포 성장을 억제하고 세포 사멸을 강력하게 유도합니다.
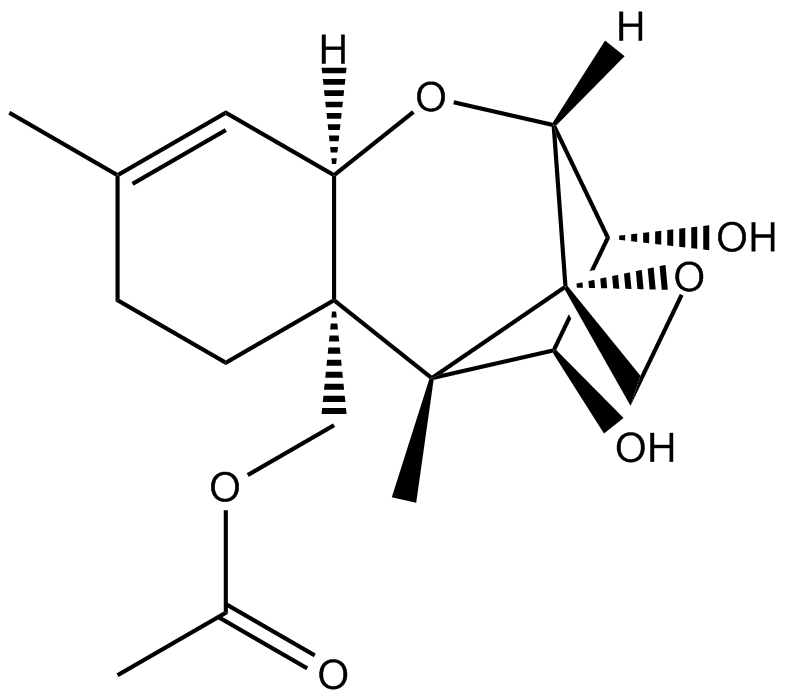
-
GC19442
15-Acetyldeoxy Nivalenol
15-Acetyldeoxy Nivalenol은 곡물에서 발견되는 고독성 트리코테센이며, deoxynivalenol의 대사산물로 HepG2 세포에 독성을 나타냅니다.

-
GC41937
15-keto-17-phenyl trinor Prostaglandin F2α ethyl amide
Bimatoprost is the Allergan trade name for 17-phenyl trinor prostaglandin F2α ethyl amide (17-phenyl trinor PGF2α ethyl amide), an F-series PG analog which has been approved for use as an ocular hypotensive drug.
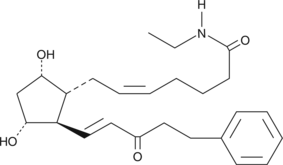
-
GC41938
15-Lipoxygenase Inhibitor 1
15-리폭시게나제 억제제 1은 IC50이 18 μ인 15-리폭시게나제의 선택적 억제제입니다. 15-리폭시게나제 억제제 1은 IC50이 각각 19.5 μM 및 19.1 μM입니다. 대두 15-리폭시게나제(SLO) 및 인간 15-리폭시게나제-1(15)-LOX-1(15)- 15-Lipoxygenase Inhibitor 1은 전립선암 연구에 잠재력이 있습니다.
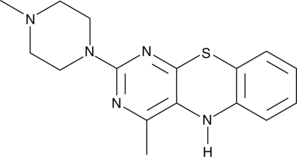
-
GC41940
15-OxoEDE
15-OxoEDE is produced by the oxidation of 15-HEDE.
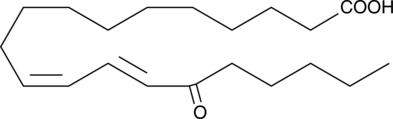
-
GC40376
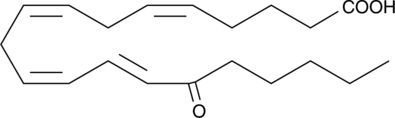
15-OxoETE
15-OxoETE is produced by oxidation of the 15-hydroxyl of 15-HETE.
-
GC41309
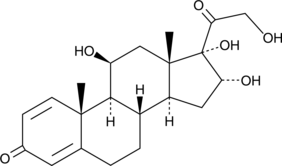
16α-hydroxy Prednisolone
16α-하이드록시 프레드니솔론은 시토크롬 P450 3A(CYP3A) 효소를 통한 글루코코르티코이드 부데소나이드의 22(R) 에피머의 입체 선택적 대사 산물입니다.
-
GC35058
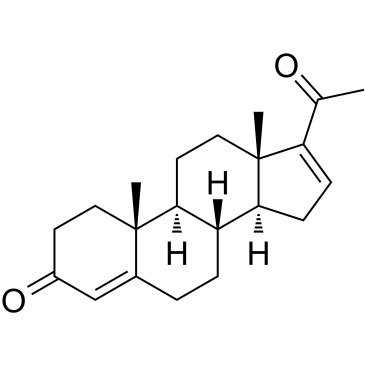
16-Dehydroprogesterone
16-디하이드로프로게스테론은 스테로이드성 프로게스틴입니다.
-
GC46451
16F16
A PDI inhibitor

-
GC45909
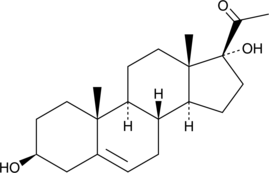
17α-hydroxy Pregnenolone
1&7#945;-하이드록시 프레그네놀론은 프레그난 스테로이드입니다.
-
GC41300
17β-hydroxy Exemestane
17β-hydroxy Exemestane is the primary active metabolite of exemestane.

-
GC41951
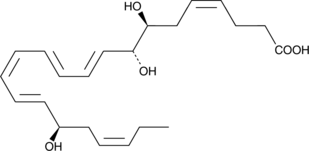
17(R)-Resolvin D1
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid and docosahexaenoic acid.
-
GC41227
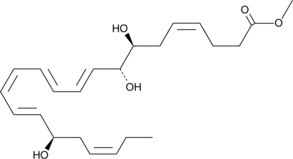
17(R)-Resolvin D1 methyl ester
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).
-
GC41952
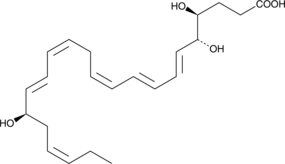
17(R)-Resolvin D4
17(R)-Resolvin D4 (17(R)-RvD4) is an aspirin-triggered epimer of RvD4 .
-
GC41208
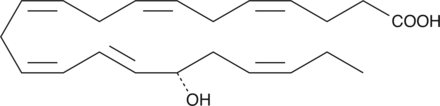
17(S)-HDHA
17(S)-HDHA is a primary mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, and mouse brain.
-
GC49356
17(S)-HDoTE
A metabolite of adrenic acid
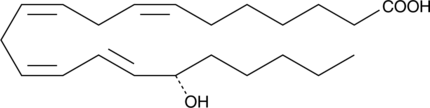
-
GC40975
17(S)-HpDHA
17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.
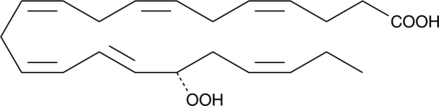
-
GC11720
17-AAG (KOS953)
An inhibitor of Hsp90
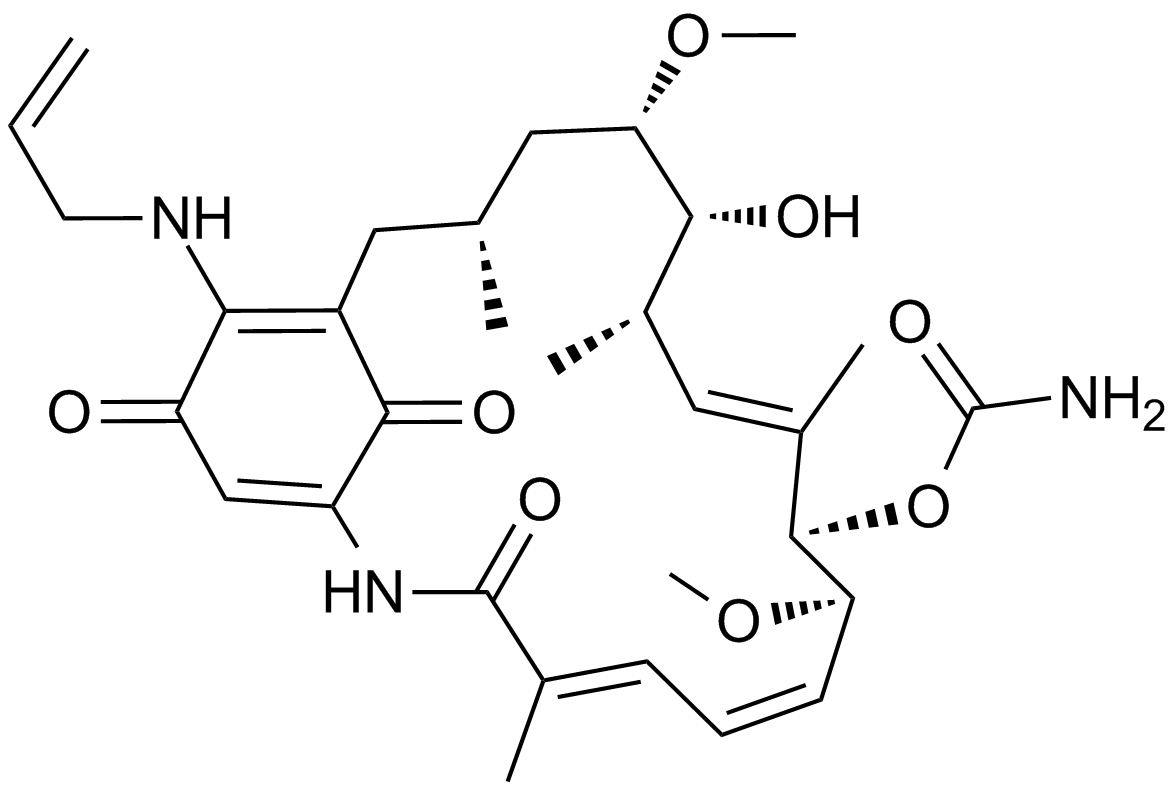
-
GC17210
17-AAG Hydrochloride
Hsp90 inhibitor,geldanamycin analogue
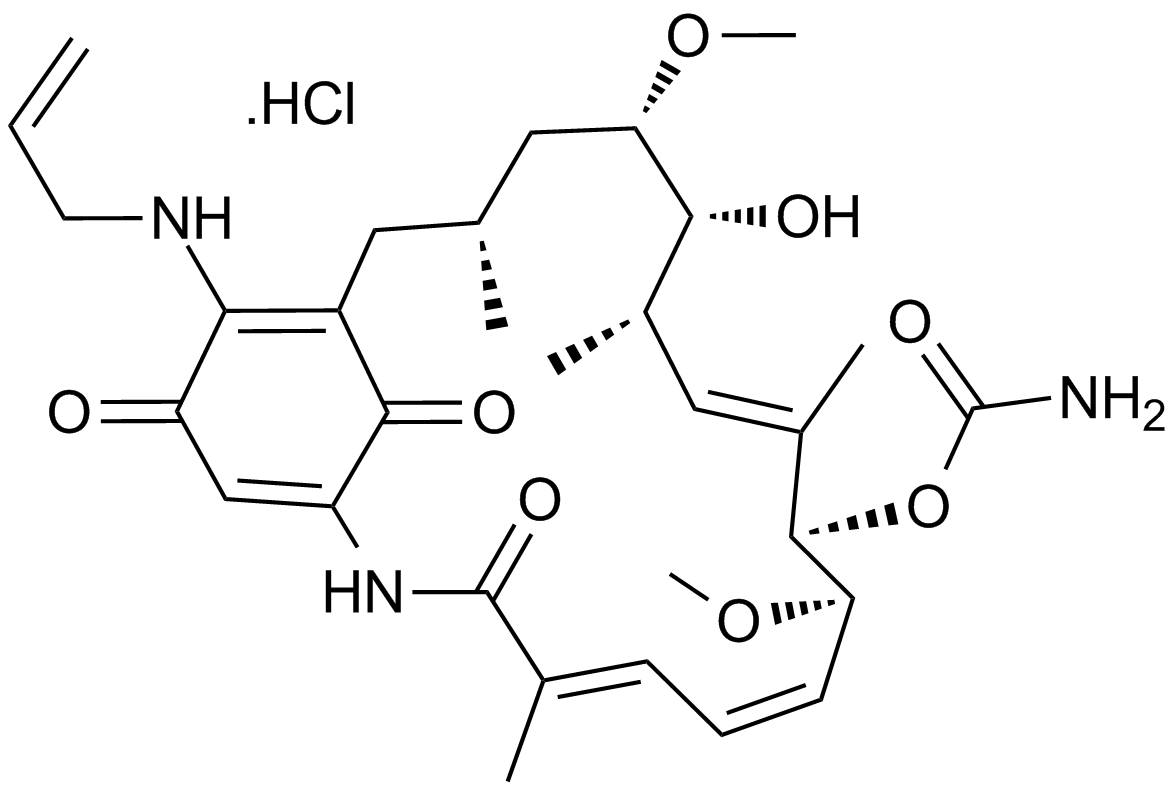
-
GC41955
17-DMAG
17-DMAG(17-DMAG)는 62 ±의 EC50으로 Hsp90에 결합하는 Hsp90의 강력한 억제제입니다. 29nM.
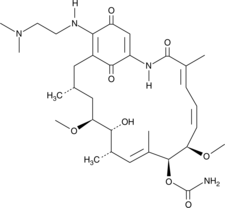
-
GC13044
17-DMAG (Alvespimycin) HCl
17-DMAG(알베스피마이신) HCl(17-DMAG 염산염; KOS-1022; BMS 826476)은 62&7#177;29nM의 EC50으로 Hsp90에 결합하는 Hsp90의 강력한 억제제입니다.

-
GC41529
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic Acid
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic acid is a metabolite of lipoxygenase-mediated oxidation of DHA that is produced endogenously by aspirin-enhanced COX-2 activity.
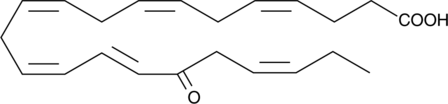
-
GC41209
17-oxo-7(Z),10(Z),13(Z),15(E),19(Z)-Docosapentaenoic Acid
Docosapentaenoic acid (DPA) is a ω-3 fatty acid found in fish oils.
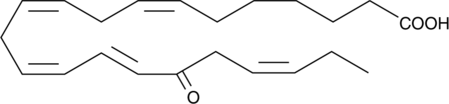
-
GC68426
17a-Hydroxypregnenolone-d3
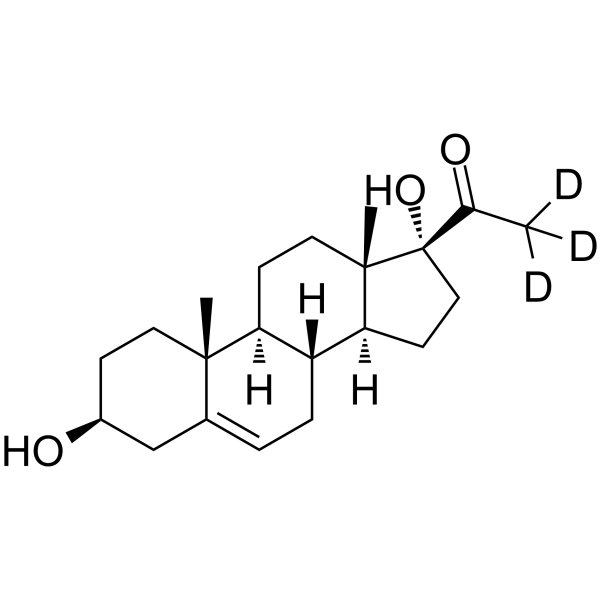
-
GC35061
18α-Glycyrrhetinic acid
18α-식이유래 화합물인 글리시레틴산은 다세포 유기체에서 장수 및 항응집 인자로 작용하는 NF-kB의 억제제이자 프로테아좀의 활성화제입니다.
-
GC41980
18-carboxy dinor Leukotriene B4
18-carboxy dinor Leukotriene B4 (18-carboxy dinor LTB4) is a β-oxidation metabolite of LTB4.
-
GC33818
18-Hydroxycorticosterone
18-히드록시코르티코스테론은 코르티코스테로이드이자 코르티코스테론의 유도체로 심각한 전해질 불균형을 유발할 수 있습니다.
-
GC63603
19-Hydroxyandrost-4-ene-3,17-dione
-
GC39296
1G244
1G244는 IC50이 각각 12nM 및 84nM인 강력한 DPP8/9 억제제입니다. 1G244는 DPPIV 및 DPPII를 억제하지 않습니다. 1G244는 다발성 골수종 세포에서 세포 사멸을 유도하고 항골수종 효과가 있습니다.
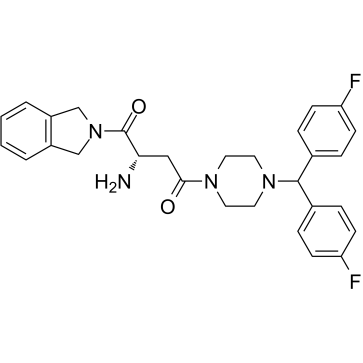
-
GC38359
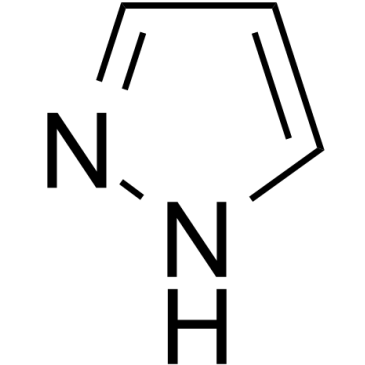
1H-pyrazole
1H-피라졸은 내인성 대사 산물입니다.
-
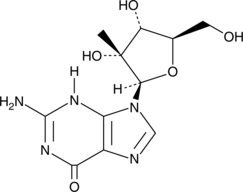
GC49823
2′-C-β-Methylguanosine
An active nucleoside metabolite of BMS-986094
-
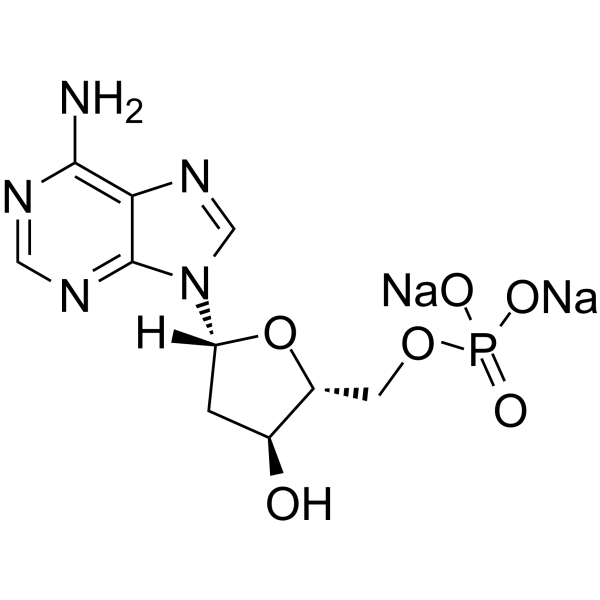
GC64738
2′-Deoxyadenosine 5′-monophosphate disodium
&2#8242;-데옥시아데노신 5′-핵산 AMP 유도체인 모노포스페이트 이나트륨은 DNA에서 발견되는 데옥시리보뉴클레오티드입니다.
-
GC62772
2’-Deoxyadenosine-5’-triphosphate trisodium
&2rsquo;-Deoxyadenosine-5’-triphosphate trisodium(dATP trisodium)은 DNA 중합효소의 기질로서 DNA 합성(또는 복제)을 위해 세포에서 사용되는 뉴클레오티드입니다.
-
GC62774
2’-Deoxyguanosine 5’-monophosphate disodium
&2rsquo;-데옥시구아노신 5’-일인산 이나트륨(5'-dGMP 이나트륨)은 핵염기로 구아닌을 갖는 모노뉴클레오티드입니다.
-
GC46508
2',2'-Difluoro-2'-deoxyuridine
An active metabolite of gemcitabine
-
GC33622
2',4'-Dihydroxyacetophenone
2',4'-디히드록시아세토페논(Resacetophenone)은 2' 및 4' 위치에 히드록시 치환기를 갖는 아세토페논입니다.
-
GC60462
2',4'-Dimethylacetophenone
-
GC33605
2'-Deoxyadenosine monohydrate
2'-데옥시아데노신 일수화물은 데옥시리보뉴클레오사이드입니다.
-
GC38258
2'-Deoxyadenosine-5'-monophosphate
핵산 AMP 유도체인 2'-데옥시아데노신 5'-모노포스페이트는 DNA에서 발견되는 데옥시리보뉴클레오티드입니다.
-
GC42150
2'-Deoxycytidine 5'-diphosphate (sodium salt hydrate)
2'-데옥시시티딘-5'-이인산(dCDP) 삼나트륨은 내인성 대사산물입니다.
-
GC17436
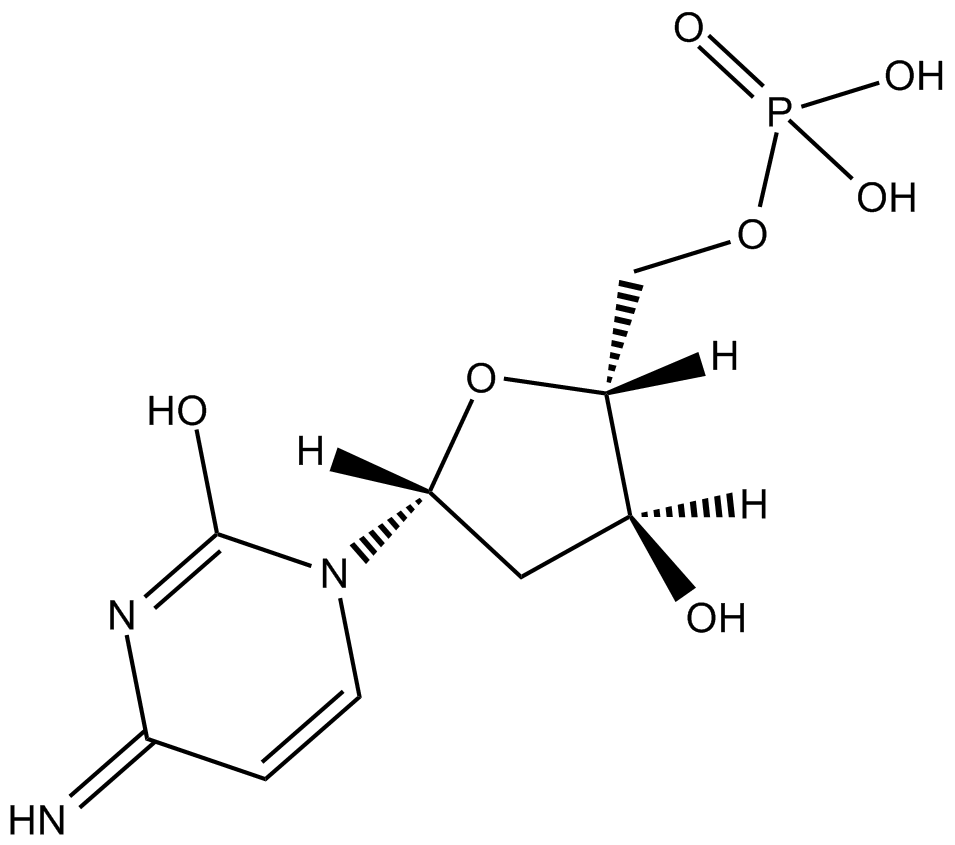
2'-Deoxycytidine-5'-monophosphoric acid
2'-Deoxycytidine-5'-monophosphoric acid는 내인성 대사 산물입니다.
-
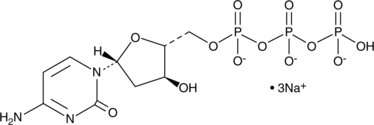
GC48440
2'-Deoxycytidine-5'-triphosphate (sodium salt)
2'-Deoxycytidine-5'-triphosphate (sodium salt) (dCTP trisodium salt)는 DNA 합성에 사용될 수 있는 뉴클레오시드 삼인산입니다.
-
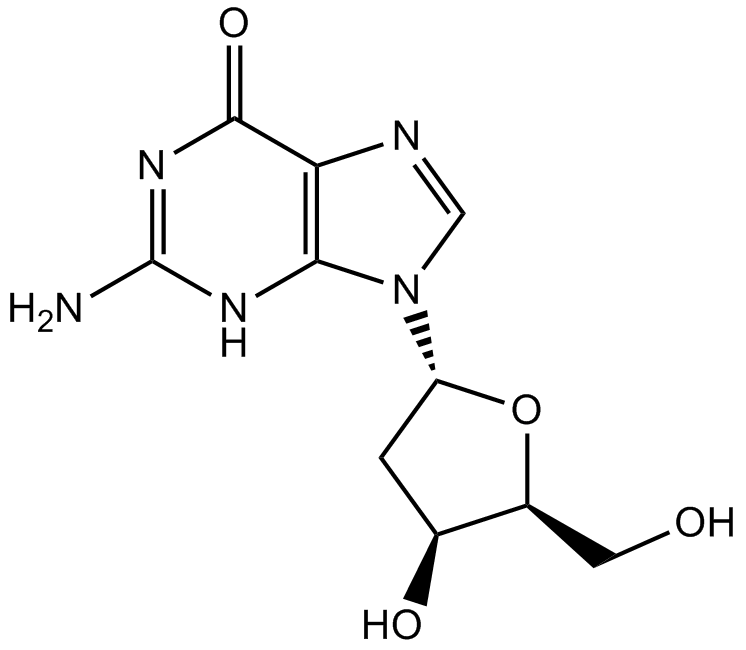
GC10897
2'-Deoxyguanosine
2'-Deoxyguanosine(Deoxyguanosine)은 N9 질소에 의해 deoxyribose의 C1 탄소에 연결된 퓨린 뉴클레오사이드 구아닌으로 구성됩니다.
-
GC38191
2'-Deoxyguanosine monohydrate
2'-Deoxyguanosine monohydrate는 내인성 대사 산물입니다.