L-689,560 |
| Catalog No.GC10696 |
potent NMDA antagonist
Products are for research use only. Not for human use. We do not sell to patients.
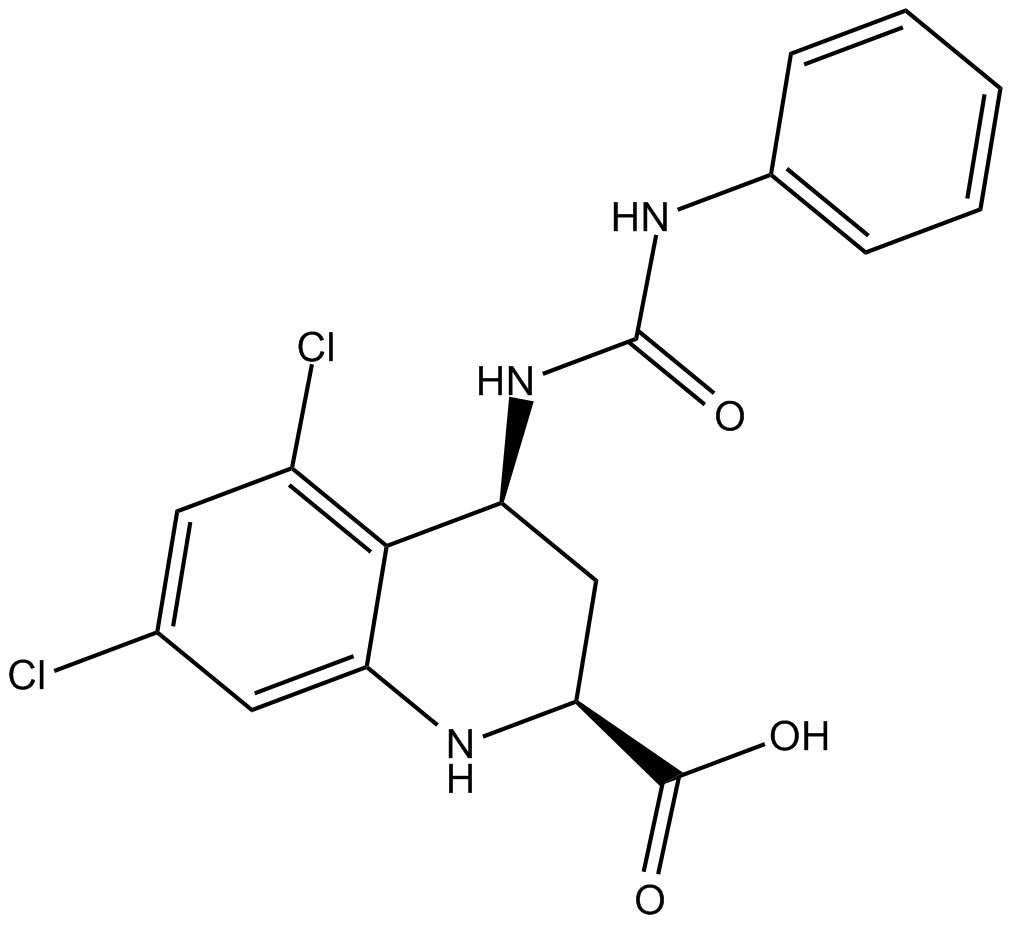
Cas No.: 139051-78-8
Sample solution is provided at 25 µL, 10mM.
Kb: 130 nM
L-689,560 is a very potent antagonist at the glycine-NMDA site. The N-methyl-D-aspartate (NMDA) subtype of excitatorynamino acid receptor has been proved adequately that its relevant antagonists can reduce ischaemic brain damage (particularly in experimental models of focal cerebral ischaemia).
In vitro: L-689560 is described as one of the most potent NMDA antagonists and [4'-3H]-L-689560 has been thought to be a highly specific radioligand for the glycine site. In consistent with the 5,7-disubstituted kynurenates, the tetrahydroquinolines are selective antagonists of glycine site NMDA, L-689560 exhibiting at least 3 orders of magnitude selectivity versus the glutamate site [1].
In vivo: MDL100748 with an ED50 of 83 mg kg-1 can prevent audiogenic seizures in susceptible mice after systemic injection. As a standard L689560, its subsequent analogues have been compared; the displacement of [3H] L689560 has often been used to displace that of [3H] glycine as an alternative assay. L701252, a quinones (the retention of a keto grouping at position 3), has been against L689560 binding (IC50 of 420 nM) and against seizures (ED50 of 4.1 mg kg-1) in DBA/2 mice. A group of sulfonamide analogues of kynurenic acid are also in active among the 2-quinolone series. Those of a series of 3,4-dihydroquinolones and tetrahydroquinolines with a nitrosubstituent at 3-position were selective antagonists at the NMDA receptor glycine site if they bore a bulky grouping in the position 4. The compound with no substitution at position 4 was proved to be one of the most effective broad-spectrum antagonists against NMDA and AMPA receptors [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1]. Leeson PD, Carling RW, Moore KW, Moseley AM, Smith JD, Stevenson G, Chan T, Baker R, Foster AC, Grimwood S, et al. 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem. 1992 May 29;35 (11): 1954-68.
[2]. Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci. 2000 Apr; 21(4):149-54.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















