L-α-Hydroxyglutaric Acid (Synonyms: 2(S)-HG, 2(S)-Hydroxyglutaric Acid, L-2-HG, L-2-Hydroxyglutaric Acid) |
| Catalog No.GC41478 |
L-α-Hydroxyglutaric Acid is an important metabolite in various domains of life.
Products are for research use only. Not for human use. We do not sell to patients.
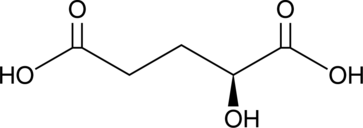
Cas No.: 13095-48-2
Sample solution is provided at 25 µL, 10mM.
L-α-Hydroxyglutaric Acid is an important metabolite in various domains of life. In mammals and plants, it is produced by lactate dehydrogenase (LDH) and malate dehydrogenase (MDH)-mediated 2-ketoglutarate (2-KG) reduction under hypoxic conditions[2]. L-α-Hydroxyglutaric Acid is an inhibitor of 2-KG dependent dioxygenases with specific pro-oncogenic capabilities[3,4].
In CD8-positive T-lymphocytes, L-α-Hydroxyglutaric Acid inhibited the production of effector cytokines and reduced cytotoxicity. In addition, when the dose of L-α-Hydroxyglutaric Acid was more than 300 μM, L-α-Hydroxyglutaric Acid inhibited cell expansion, increased cell apoptosis, decreased IFN-γ secretion, but increased IL-2 secretion[6].This compound was identified to aid the proliferation and antitumorigenic abilities of CD8+ T-lymphocytes, to contribute to relieving the cellular reductive stress[5].
When examined the effect of L-α-Hydroxyglutaric Acid, at concentrations varying from 1 to 5 mM, on CK activity in total homogenates from cerebellum, cerebral cortex, skeletal muscle and cardiac muscle. L-α-Hydroxyglutaric Acid did not alter tCK activities from cerebral cortex, skeletal muscle, and cardiac muscle, but significantly inhibited the enzyme activity from cerebellum at all concentrations tested. The inhibition of Mi-CK activity by L-α-Hydroxyglutaric Acid is non-competitive. The Km calculated was 2.52±0.74 mM. The Ki value was calculated by the method of which provides a simple way of determining the inhibition constant (Ki) for non-competitive inhibitors. The Ki value calculated was 11.13±3.71 mM for L-α-Hydroxyglutaric Acid [1].
Besides, in Zebrafish, L-α-Hydroxyglutaric Acid-induced apoptosis was caused by oxidation[7].
References:
[1]: da Silva CG, Bueno AR, et,al. L-2-hydroxyglutaric acid inhibits mitochondrial creatine kinase activity from cerebellum of developing rats. Int J Dev Neurosci. 2003 Jun;21(4):217-24. doi: 10.1016/s0736-5748(03)00035-2. PMID: 12781789.
[2]: Oldham WM, Clish CB, et,al. Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell Metab. 2015 Aug 4;22(2):291-303. doi: 10.1016/j.cmet.2015.06.021. Epub 2015 Jul 23. PMID: 26212716; PMCID: PMC4526408.
[3]: Xu W, Yang H, et,al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011 Jan 18;19(1):17-30. doi: 10.1016/j.ccr.2010.12.014. PMID: 21251613; PMCID: PMC3229304.
[4]: Chowdhury R, Yeoh KK, et,al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011 May;12(5):463-9. doi: 10.1038/embor.2011.43. Epub 2011 Apr 1. PMID: 21460794; PMCID: PMC3090014.
[5]: Tyrakis PA, Palazon A, et,al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016 Dec 8;540(7632):236-241. doi: 10.1038/nature20165. Epub 2016 Oct 26. PMID: 27798602; PMCID: PMC5149074.
[6]: Tyrakis PA, Palazon A, et,al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016 Dec 8;540(7632):236-241. doi: 10.1038/nature20165. Epub 2016 Oct 26. PMID: 27798602; PMCID: PMC5149074.
[7]:Parng C, Ton C, et,al. A zebrafish assay for identifying neuroprotectants in vivo. Neurotoxicol Teratol. 2006 Jul-Aug;28(4):509-16. doi: 10.1016/j.ntt.2006.04.003. Epub 2006 Jun 30. PMID: 16814516.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















