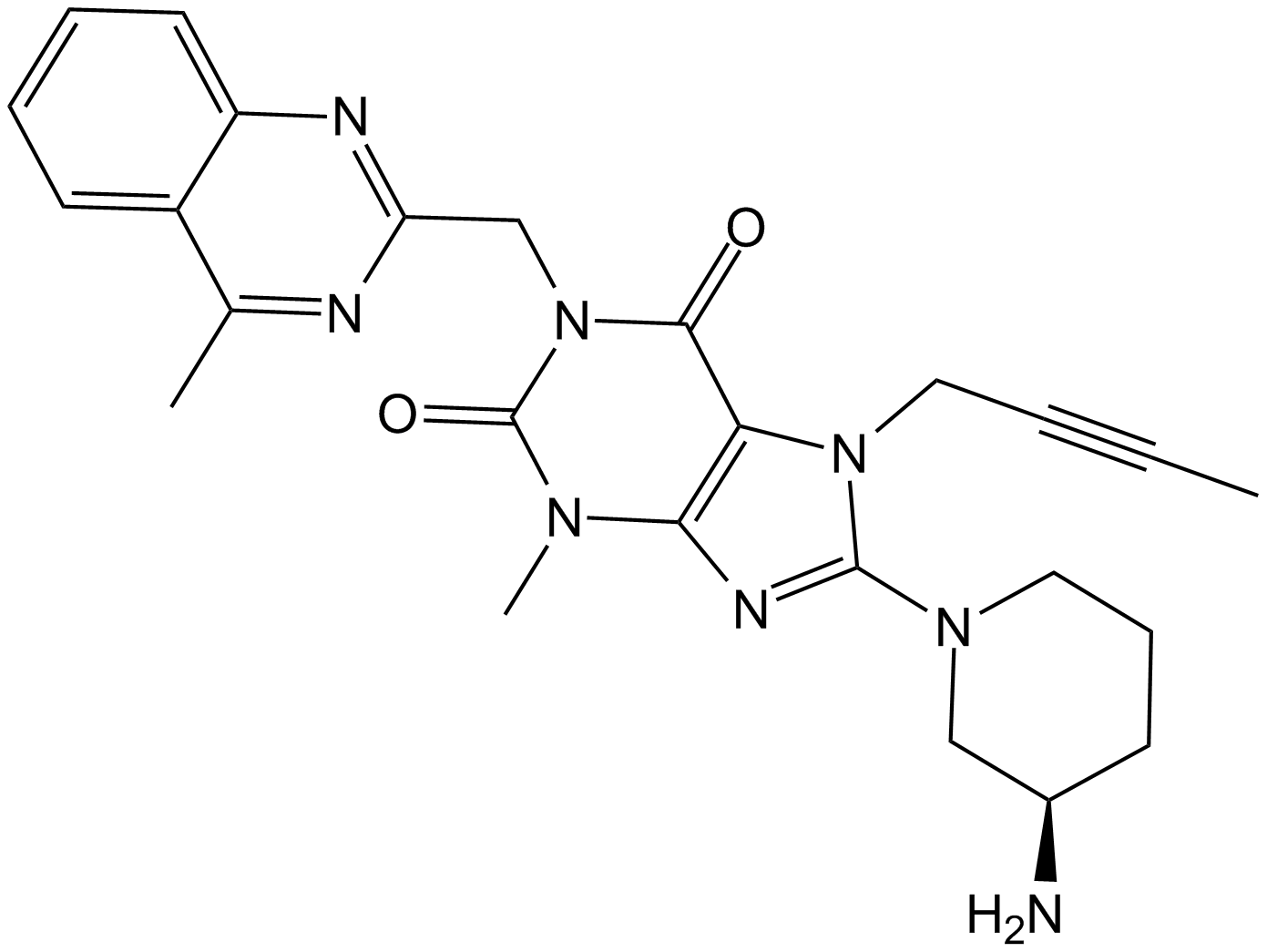
Linagliptin (BI-1356) (Synonyms: BI-1356) |
| Catalog No.GC14827 |
A potent DPP-4 inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 668270-12-0
Sample solution is provided at 25 µL, 10mM.
BI1356 is a potent and competitive DPP-4 inhibitor, which exhibited DPP-4 inhibiting activity in several independent tests with IC50 values of 0.4, 0.5, 0.9, and 1.1nM.[1]
DPP-4 is an N-terminal dipeptidyl exopeptidase existing as a membrane-bound protein and also as a soluble protein in plasma. It plays a major role in the degradation of incretins such as GLP-1 which is of great importance in the process of glucose metabolism. Under physiological conditions, GLP-1 is truncated by DPP-4 rapidly, which is located on the capillary endothelium proximal to the L-cells where GLP-1 is secreted in the ileum. GLP-1 could sensitize β-cells to glucose stimulation,consequently increasing intracellular cAMP concentrations in β-cells and accelerating and augmenting insulin response to absorb glucose. By being measured at the substrate at the binding site, BI1356 inhibits the DPP-4 enzyme.[1]
DPP-4 was extracted from confluent Caco-2 cells to determine the inhibition activity of BI1356. Assays were performed by mixing the inhibitor solution with substrates and the Caco-2 cell extract, which illustrated BI1356 inhibited DPP-4 with IC 50 values of 0.4, 0.5, 0.9, and 1.1 nM. BI1356 also possesses a very significant selectivity for DPP-4 relative to other dipeptidyl peptidases aminopeptidases N and P, prolyloligopeptidase, and the proteases trypsin, plasmin, and thrombin.[1]
An in vivo evaluation showed that BI1356 dose-dependently inhibited the DPP-4 enzyme in plasma within 30 min of administration. Separate doses ranging from 1 to 10 mg/kg achieved significant inhibition activity of DPP-4, which also showed persistent DPP-4 inhibition activity. The ED50 value for inhibition of plasma DPP-4 activity was calculated to be 0.9 mg/kg 24 h post dose. In a clinical study, BI1356 produced a remarkable, clinically meaningful and persistent improvement in glycaemic control, in accordance with enhanced parameters of β-cell function. Patients treated with BI1356 were more likely to achieve a reduction in HbA1c of ≥ 0.5% comparing control.[1,2]
References:
1.Thomas L, Eckhardt M, Langkopf E, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3, 7-dihydro-purine-2, 6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors[J]. Journal of Pharmacology and Experimental Therapeutics, 2008, 325(1): 175-182.
2.Del Prato S, Barnett A H, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of β‐cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial[J]. Diabetes, Obesity and Metabolism, 2011, 13(3): 258-267.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















