Leukotrienes
Products for Leukotrienes
- Cat.No. Product Name Information
-
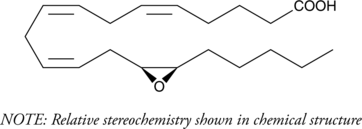
GC40430
(±)14(15)-EET
(±)14(15)-EET is biosynthesized in rat and rabbit liver microsomes by CYP450.
-
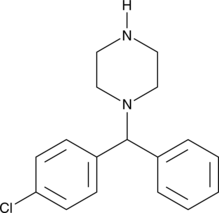
GC49294
1-(4-Chlorobenzhydryl)piperazine
An inactive metabolite of meclizine and chlorcyclizine
-
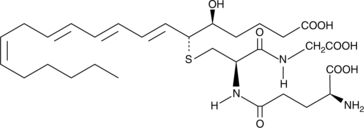
GC41144
11-trans Leukotriene C4
11-trans Leukotriene C4 (11-trans LTC4) is a C-11 double bond isomer of LTC4.
-
GC41147
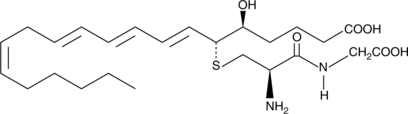
11-trans Leukotriene D4
11-trans Leukotriene D4 (11-trans LTD4) is a C-11 double bond isomer of LTD4.
-
GC41149
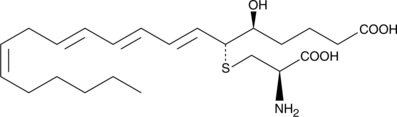
11-trans Leukotriene E4
Slow isomerization of the C-11 double bond of LTE4 leads to the formation of 11-trans LTE4.
-
GC41123
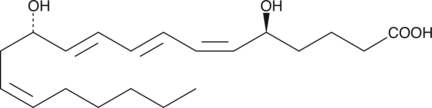
12-epi Leukotriene B4
Leukotriene B4 (LTB4) compounds are produced by both enzymatic and non-enzymatic processes.
-
GC41096
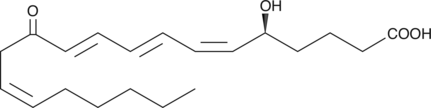
12-oxo Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-LO pathway.
-
GC41100
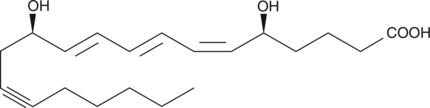
14,15-dehydro Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-lipoxygenase pathway.
-
GC41320
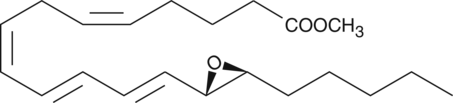
14,15-Leukotriene A4 methyl ester
14,15-Leukotriene A4 (14,15-LTA4) methyl ester is an esterified form of 14,15-LTA4, a leukotriene isomer formed from arachidonic acid by 15-lipoxygenase (15-LO) that inhibits LTA4 hydrolase.
-
GC41145
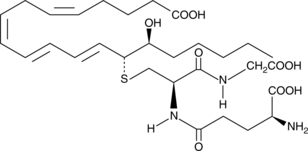
14,15-Leukotriene C4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
-
GC41148
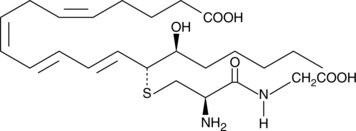
14,15-Leukotriene D4
14,15-Leukotriene D4 (14,15-LTD4) is a member of an alternate class of LTs synthesized by a pathway involving the dual actions of 15- and 12-lipoxygenases (15- and 12-LOs) on arachidonic acid via 15-HpETE and 14,15-LTA4 intermediates.
-
GC41150
14,15-Leukotriene E4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
-
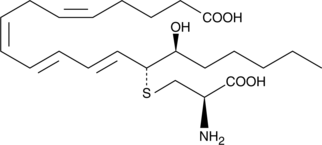
GC41980
18-carboxy dinor Leukotriene B4
18-carboxy dinor Leukotriene B4 (18-carboxy dinor LTB4) is a β-oxidation metabolite of LTB4.
-
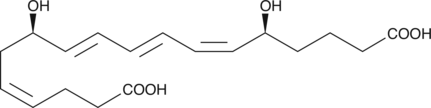
GC42082
20-carboxy Leukotriene B4
20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils.
-
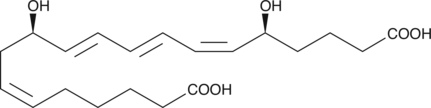
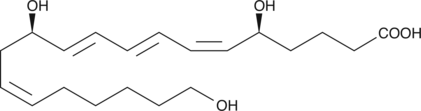
GC41421
20-hydroxy Leukotriene B4
20-hydroxy LTB4 is a metabolite of LTB4 in human neutrophils.
-
GC52413
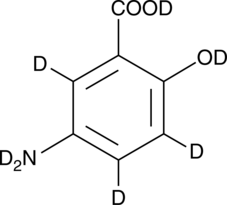
5-Aminosalicylic Acid-d7
An internal standard for the quantification of 5-aminosalicylic acid
-
GC49591
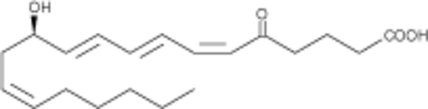
5-oxo Leukotriene B4
A byproduct in 12-oxo LTB4 synthesis
-
GC41134
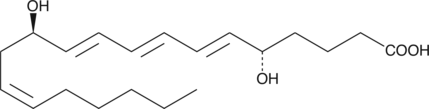
6-trans Leukotriene B4
6-trans LTB4 is produced by the non-enzymatic hydrolysis of LTA4.
-
GC41135
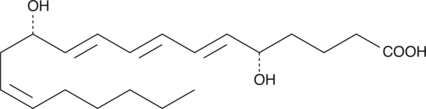
6-trans-12-epi Leukotriene B4
6-trans-12-epi Leukotriene B4 (LTB4) is a non-enzymatic hydrolysis product of LTA4.
-
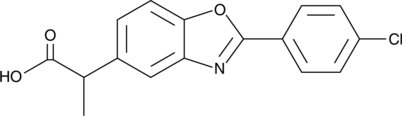
GC49836
Benoxaprofen
Benoxaprofen (LRCL 3794) is a potent and long-acting anti-inflammatory and antipyretic compound.
-
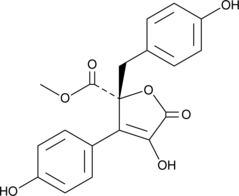
GC46105
Butyrolactone II
A fungal metabolite
-
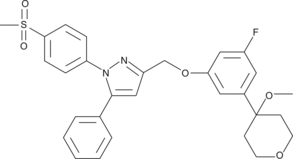
GC43153
CAY10416
Dual cyclooxygenase-2 (COX-2)/5-lipoxygenase (5-LO) inhibitors are potential therapeutic agents for inflammatory diseases and for prostate cancer.
-
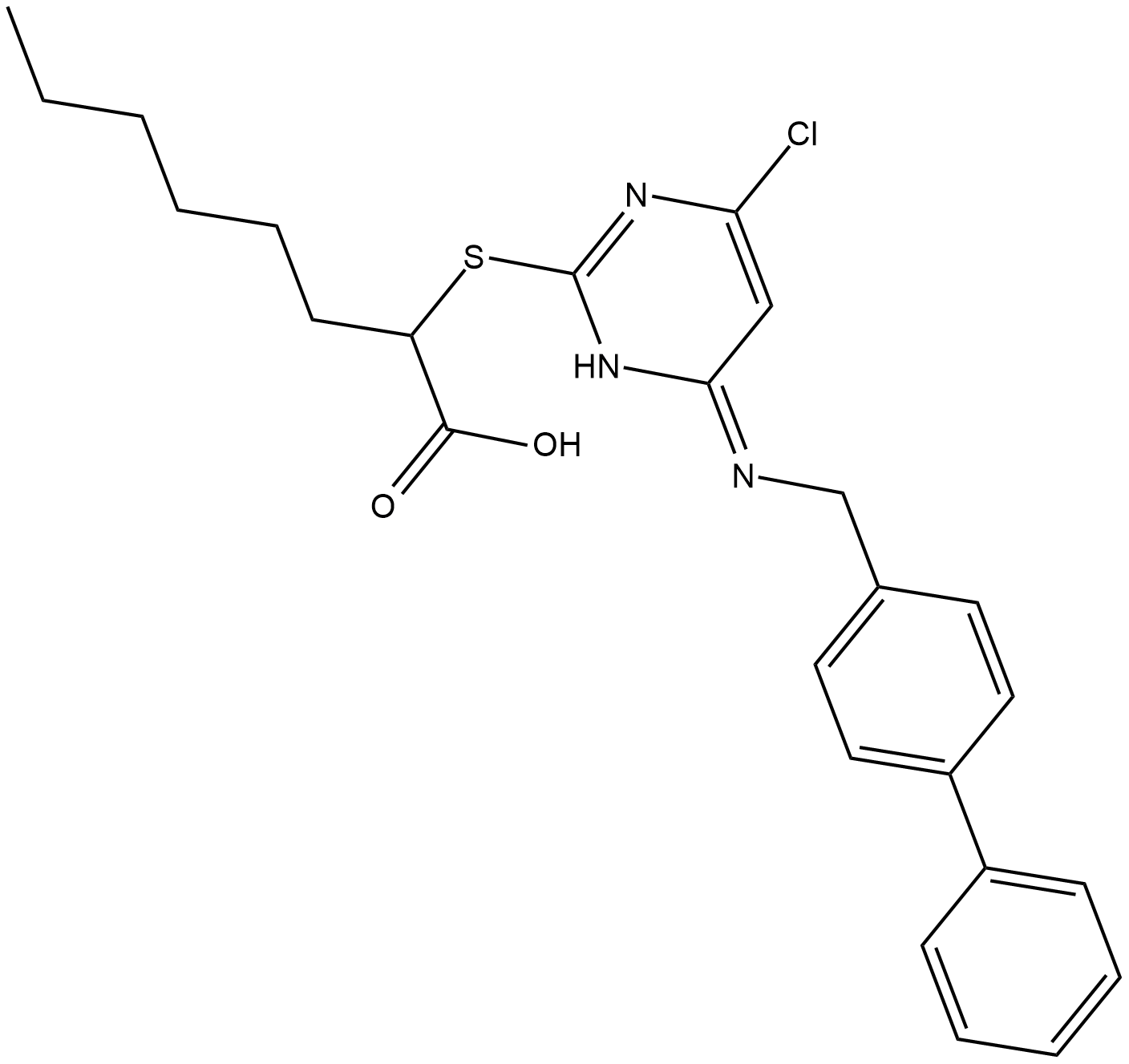
GC18688
CAY10589
Microsomal Prostaglandin E2 Synthase-1 (mPGES-1), with cyclooxygenase-2 (COX-2), synthesizes PGE2, which is directly involved in signaling during inflammation, fever and pain.
-
GC18877
CAY10606
5-Lipoxygenase (5-LO) initiates the synthesis of leukotrienes (LTs) from arachidonic acid, primarily in certain leukocyte populations.
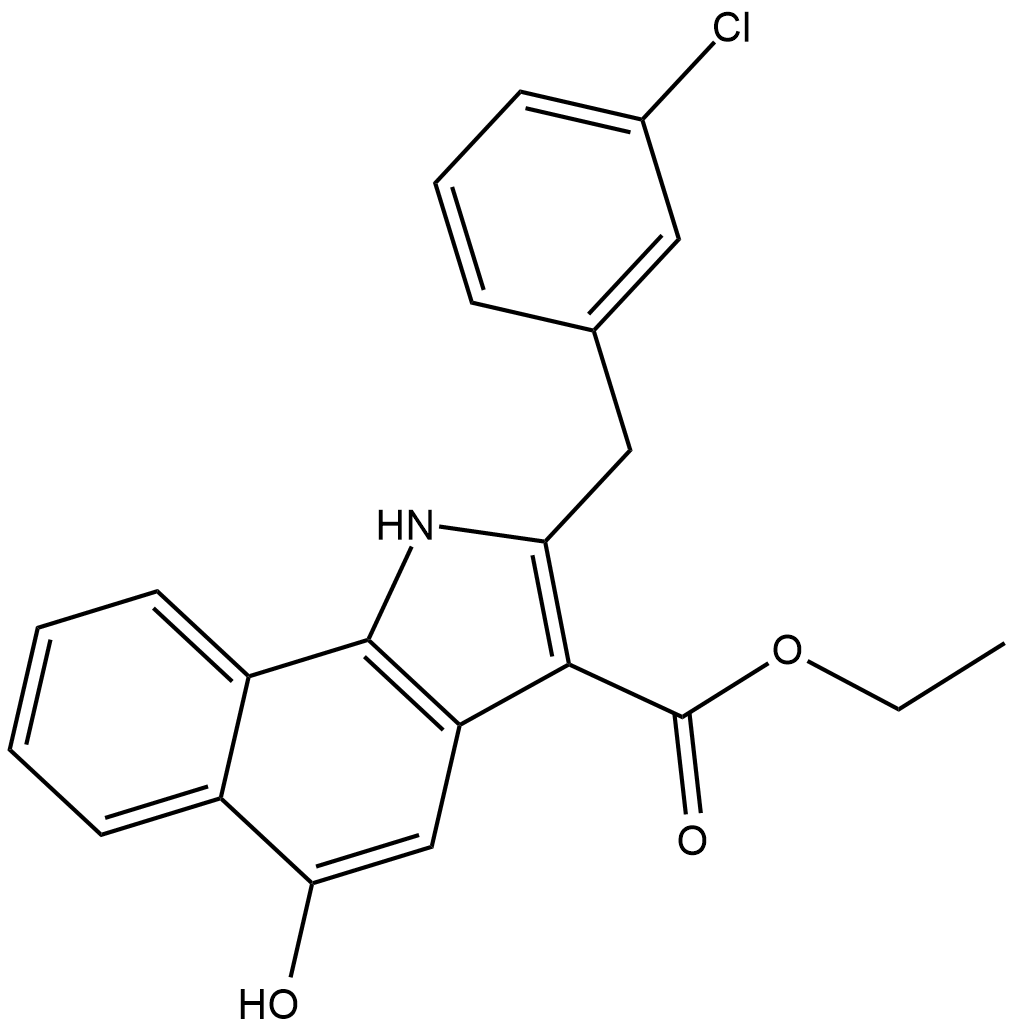
-
GC18480
CAY10649
5-Lipoxygenase (5-LO) catalyzes the biosynthesis of leukotrienes, which are involved in a variety of inflammatory responses, including neutrophil chemotaxis, vascular permeability, and smooth muscle contraction.
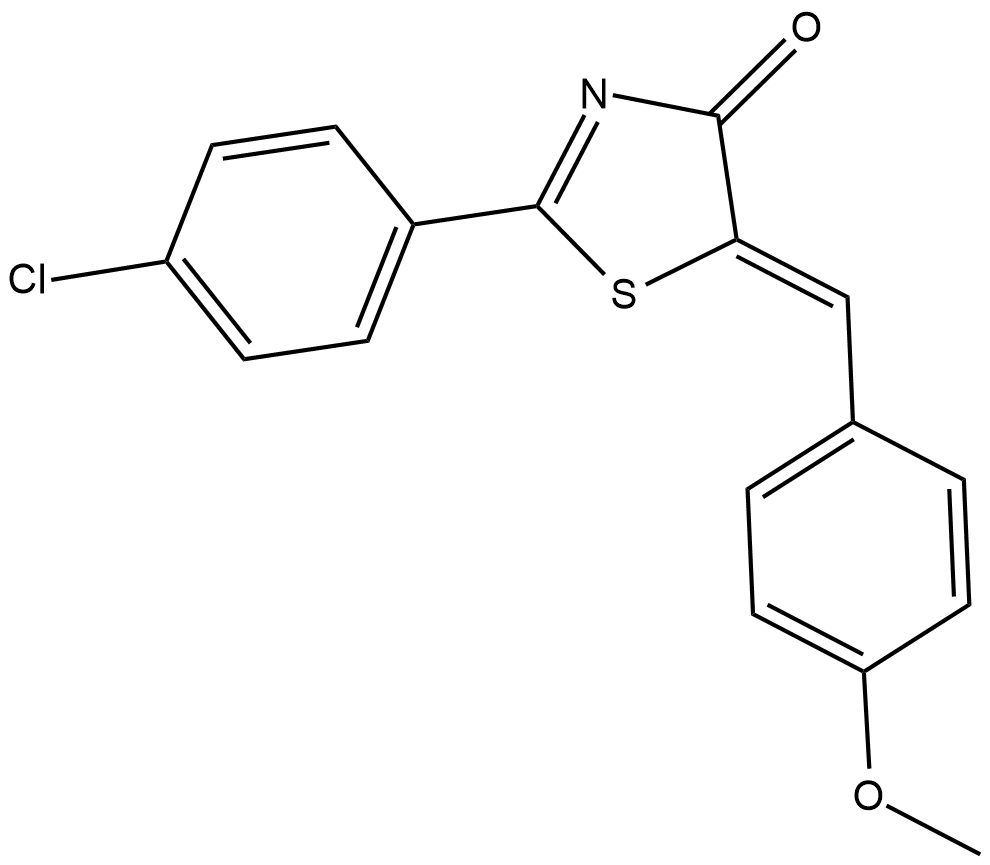
-
GC52307
CAY10790
A CysLT1 receptor antagonist and GPBAR1 agonist
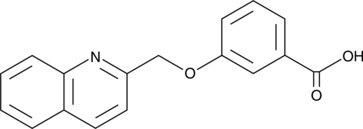
-
GC52016
Cetirizine N-oxide
An oxidative degradation product of cetirizine
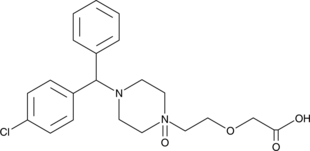
-
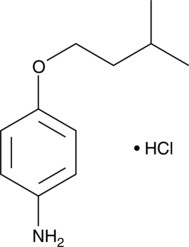
GC43319
CP 24,879 (hydrochloride)
Essential fatty acid deficiency symptoms include immune system depression and a general state of inflammatory inhibition.
-
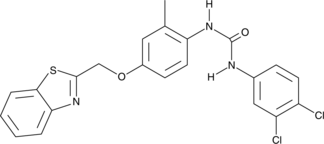
GC43453
Diflapolin
Diflapolin is a dual inhibitor of 5-lipoxygenase-activating protein (FLAP) and soluble epoxide hydrolase (sEH).
-
GC49471
LAMP1 Monoclonal Antibody (Clone Ly1C6)
For immunochemical analysis of LAMP1

-
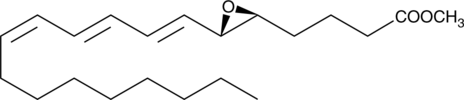
GC40840
Leukotriene A3 methyl ester
Biosynthesis of LTA3 occurs from 5,8,11-eicosatrienoic acid via the 5-LO pathway and it is the putative intermediate in the biosynthesis of 3-series leukotrienes.
-
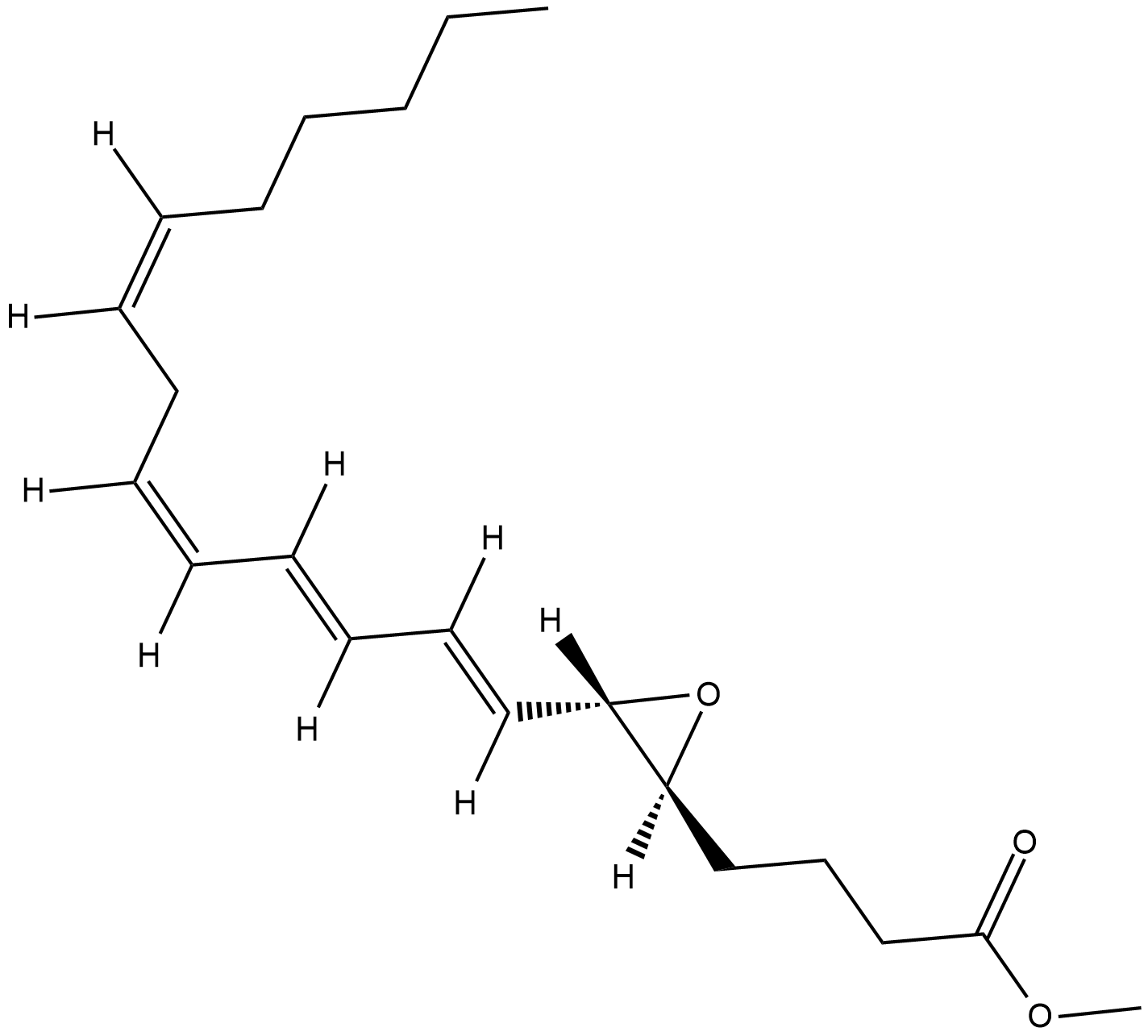
GC18835
Leukotriene A4 methyl ester
Leukotriene A4 (LTA4) is synthesized in mast cells, eosinophils, and neutrophils from arachidonic acid by 5-lipoxygenase (5-LO), which exhibits both lipoxygenase and LTA4 synthase activities.
-
GC40278
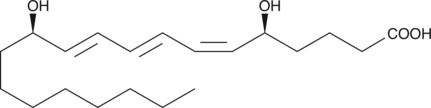
Leukotriene B3
LTB3 is the LTA hydrolase metabolite of LTA3 in the leukotriene biosynthetic pathway.
-
GC44052
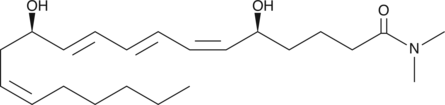
Leukotriene B4 dimethyl amide
LTB4 dimethyl amide is a moderate inhibitor of LTB4-induced degranulation of human neutrophils (Ki = 130 nM) and lysozyme release from rat PMNL.
-
GC44053
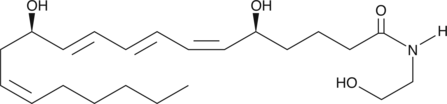
Leukotriene B4 Ethanolamide
The effects of Leukotriene B4 (LTB4) are mediated by two known receptors, BLT1 and BLT2.
-
GC40631
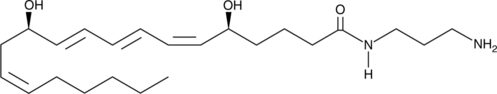
Leukotriene B4-3-aminopropylamide
The effects of leukotriene B4 (LTB4) are mediated by two receptors, BLT1 and BLT2.
-
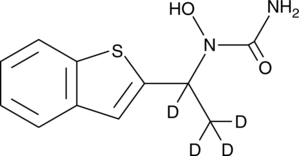
GC47556
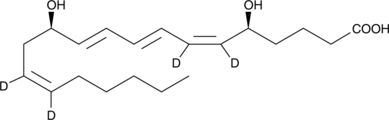
Leukotriene B4-d4
A quantitative analytical standard guaranteed to meet MaxSpec® identity, purity, stability, and concentration specifications
-
GC49597
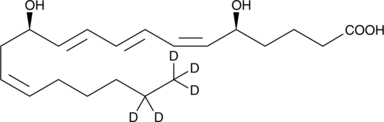
Leukotriene B4-d5
An internal standard for the quantification of LTB4
-
GC41107
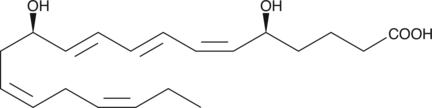
Leukotriene B5
Leukotriene B5 (LTB5) is a leukotriene with diverse biological activities.
-
GC44054
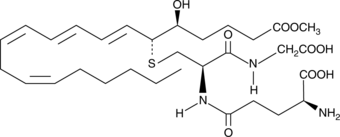
Leukotriene C4 methyl ester
Leukotriene C4 (LTC4) is the parent cysteinyl-leukotriene produced by the LTC4 synthase-catalyzed conjugation of glutathione to LTA4.
-
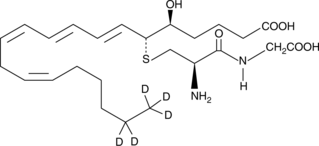
GC47559
Leukotriene D4-d5
A quantitative analytical standard guaranteed to meet MaxSpec® identity, purity, stability, and concentration specifications
-
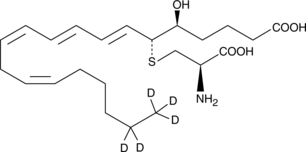
GC47560
Leukotriene E4-d5
A quantitative analytical standard guaranteed to meet MaxSpec® identity, purity, stability, and concentration specifications
-
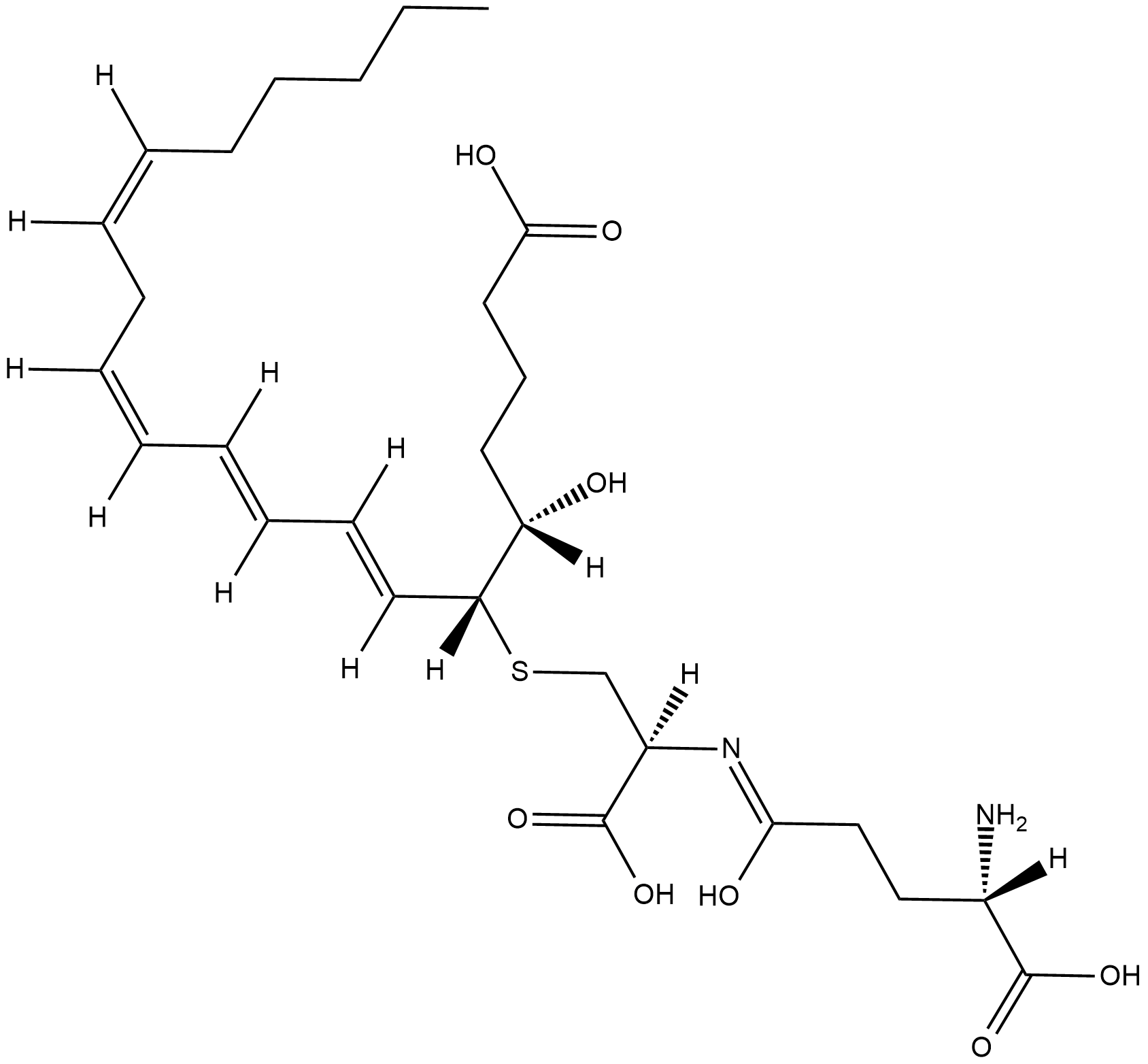
GC18845
Leukotriene F4
LTF4 is a cysteinyl-leukotriene produced in vitro, but not reported to date in vivo.
-
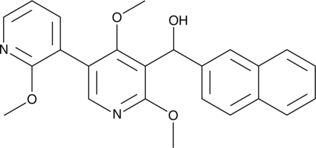
GC47570
Lipoxygenin
An inhibitor of 5-LO
-
GC47575
Loratadine-d5
An internal standard for the quantification of loratadine
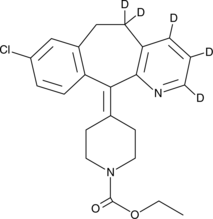
-
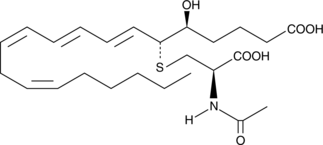
GC44295
N-acetyl Leukotriene E4
N-acetyl LTE4 is the major inactive metabolite of LTE4 found in bile.
-
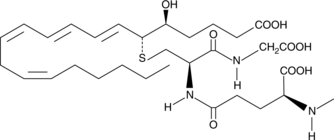
GC44415
N-methyl Leukotriene C4
Produced by neutrophils, macrophages, mast cells, and by transcellular metabolism in platelets, leukotriene C4 (LTC4) is the parent cysteinyl leukotriene formed by the LTC4 synthase-catalyzed conjugation of glutathione to LTA4.
-
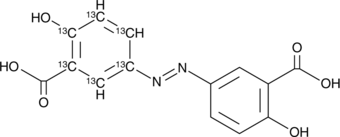
GC49098
Olsalazine-13C6
An internal standard for the quantification of olsalazine
-
GC18453
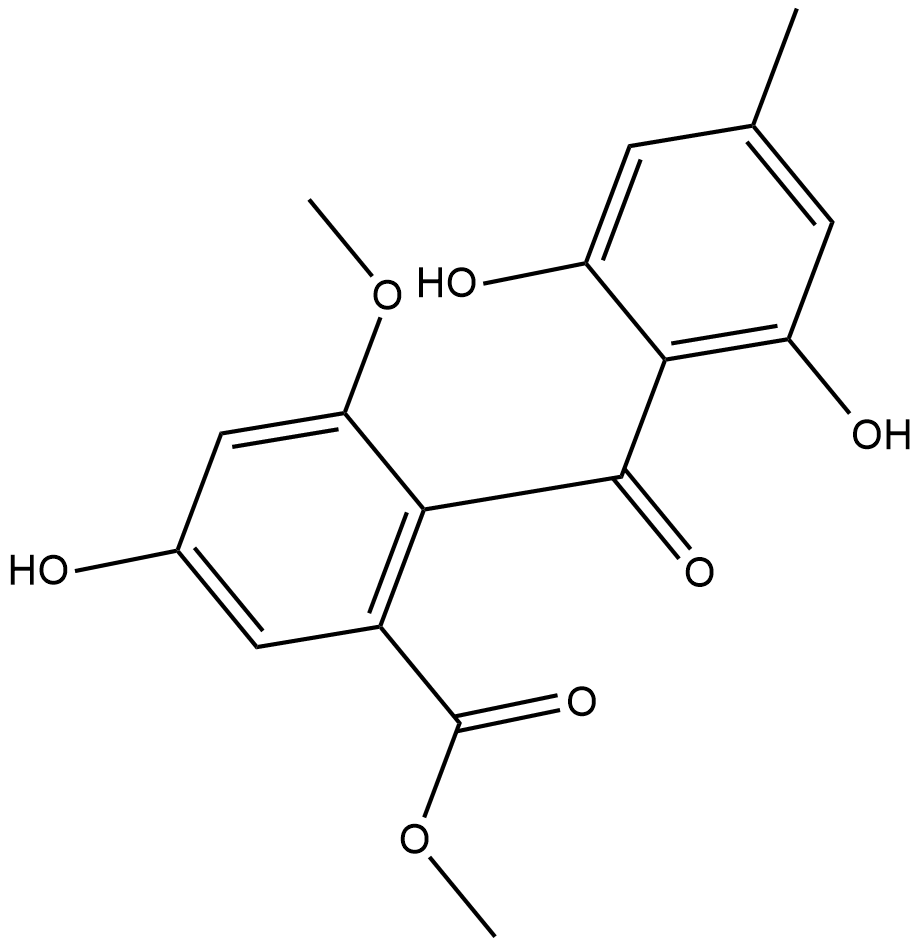
Sulochrin
Sulochrin is a fungal metabolite produced by A.
-
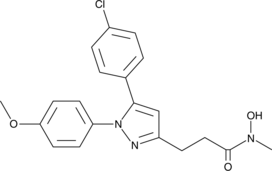
GC49386
Tepoxalin
A dual inhibitor of COX and 5-LO
-
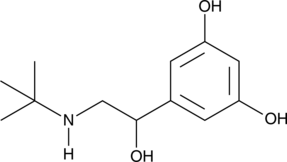
GC45993
Terbutaline
A β2-adrenergic receptor agonist
-
GC48267
Zileuton-d4
An internal standard for the quantification of zileuton