LMK 235 |
| Catalog No.GC13237 |
A selective inhibitor of HDAC4 and HDAC5
Products are for research use only. Not for human use. We do not sell to patients.
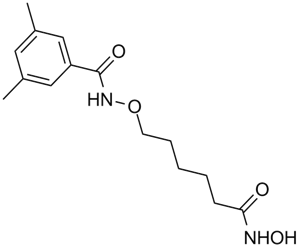
Cas No.: 1418033-25-6
Sample solution is provided at 25 µL, 10mM.
Description:
IC50: cytotoxicity IC50 (490 nm for A2780; 320 nm for A2780 CisR); HDAC inhibition (650 nM for A2780; 320 nm for A2780 CisR)
Histone deacetylases (EC 3.5.1.98, HDAC) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on a histone, allowing the histones to wrap the DNA more tightly. Histone deacetylase inhibitors (HDIs) have a long history of use in psychiatry and neurology as mood stabilizers and anti-epileptics, for example, valproic acid. In more recent times, HDIs are being studied as a mitigator or treatment for neurodegenerative diseases.
In vitro: LMK235 showed similar effects compared to vorinostat on inhibition of cellular HDACs in a pan-HDAC assay but enhanced cytotoxic effects against the human cancer cell lines A2780, Cal27, Kyse510, and MDA-MB231. LMK235 shows nanomolar inhibition of HDAC4 and HDAC5, whereas vorinostat and TSA inhibit HDAC4 and HDAC5 in the higher micromolar range. In contrast to vorinostat, LMK235 showed a novel HDAC isoform selectivity profile with preference for HDAC4 and HDAC5, which are inhibited with low nanomolar IC50 values [1].
In vivo: In a mouse in-vivo study, synergistic inhibition was demonstrated for LMK235 and Cytarabine in proliferation assays and in colony formation assays. These findings demonstrate that in vivo RNAi screening for therapeutic efficacy is feasible. HDAC4 might be an important target to enhance efficacy of anti-leukemic drugs [2].
Clinical trial: LMK235 is currently in the preclinical development and no clinical trial is ongoing.
References:
[1] Marek L, Hamacher A, Hansen FK, Kuna K, Gohlke H, Kassack MU, Kurz T. Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J Med Chem. 2013;56(2):427-36.
[2] Stephanie Lettermann, Konstantin Agelopoulos, Christian Rohde, Beate Lindtner, Linda Marek, Georg Hempel, Matthias Stelljes, Wolfgang E. Berdel, Thomas Kurz and Carsten Müller-Tidow. In Vivo RNAi Screening Identifies HDAC4 As a Mediator Of Chemoresistance In Acute Myeloid Leukemia. 55th ASH Annual Meeting and Exposition (2013) (https://ash.confex.com/ash/2013/webprogram/Paper60036.html)
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




