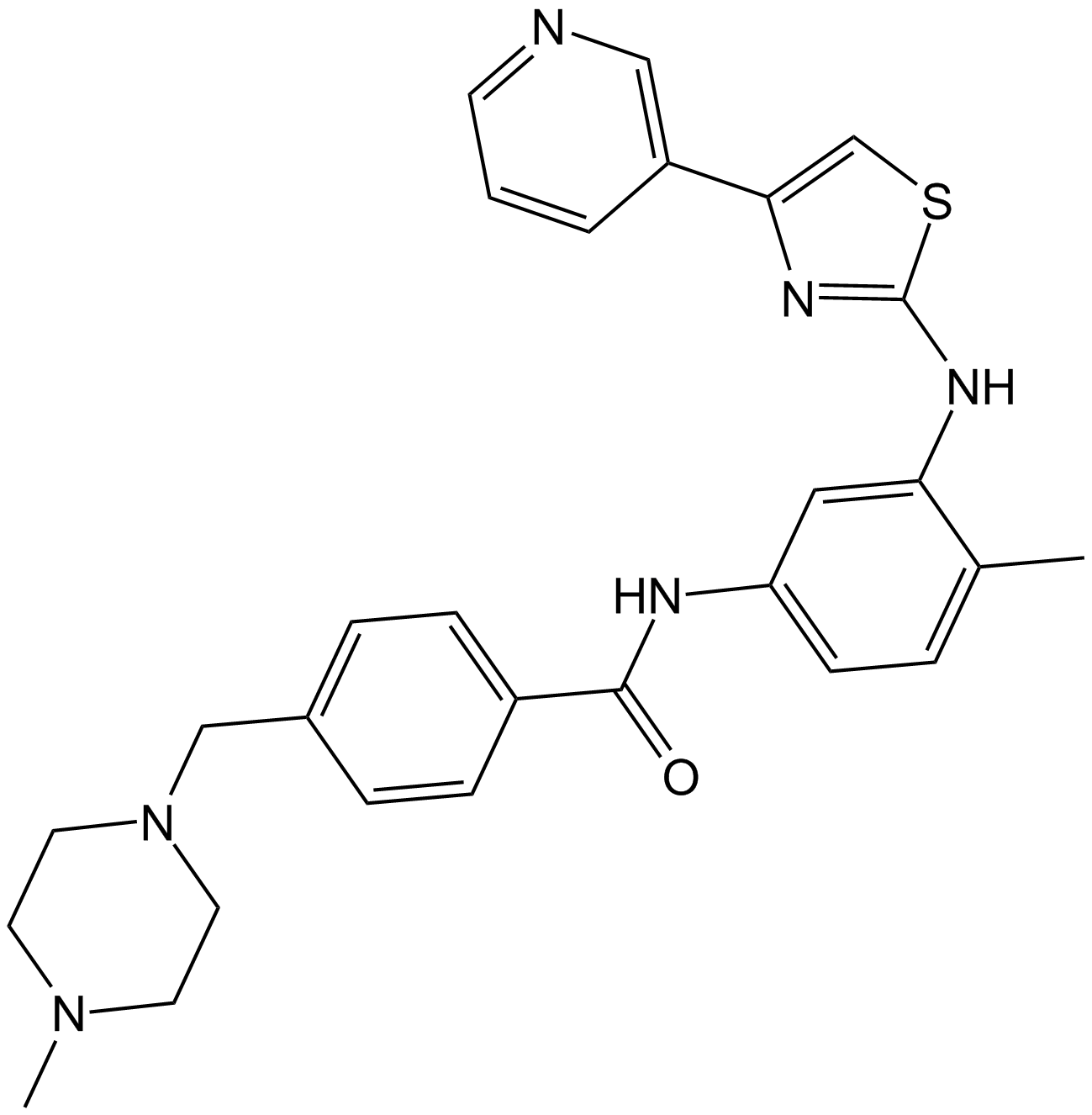
Masitinib (AB1010) (Synonyms: AB-1010, Masican, Masiviera) |
| Catalog No.GC13410 |
Masitinib (AB1010) (AB1010) is a potent, orally bioavailable, and selective inhibitor of c-Kit (IC50=200 nM for human recombinant c-Kit). It also inhibits PDGFRα/β (IC50s=540/800 nM), Lyn (IC50= 510 nM for LynB), Lck, and, to a lesser extent, FGFR3 and FAK. Masitinib (AB1010) (AB1010) has anti-proliferative, pro-apoptotic activity and low toxicity.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 790299-79-5
Sample solution is provided at 25 µL, 10mM.
The stem cell factor receptor (KIT) is a therapeutic target for the cancer, mastocytosis, and inflammatory diseases treatment. Masitinib (AB1010) is a novel phenylaminothiazole-type tyrosine kinase inhibitor that targets KIT.
In vitro: Masitinib potently inhibited the intracellular kinase Lyn and recombinant PDGFR as well as fibroblast growth factor receptor 3. Moreover, masitinib showed weak inhibition of ABL and c-Fms and was inactive against a variety of other tyrosine and serine/threonine kinases. These findings suggest that Masitinib (AB1010) will exhibit a better safety profile than other tyrosine kinase inhibitors; in fact, masitinib-induced genotoxicity or cardiotoxicity has not been observed in animal so far [1].
In vivo: In vivo, masitinib blocked tumour growth in mice with subcutaneous grafts of Ba/F3 cells expressing a juxtamembrane KIT mutant. We found that tumour growth was blocked following 5 days of treatment with masitinib. After withdrawal of masitinib treatment after day 5, tumour growth was once again evident [1].
Clinical trial: A Phase II study of oral masitinib mesilate in imatinib-naïve patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). Thirty patients were enrolled with a median follow-up of 34 months. Masitinib appears to be effective as a first-line treatment of advanced GIST with comparable results to imatinib in terms of safety and response. PFS and OS results data show promise that masitinib may provide sustainable benefits [2].
Reference:
[1] Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, Borge L, Hajem B, Lermet A, Sippl W, Voisset E, Arock M, Auclair C, Leventhal PS, Mansfield CD, Moussy A, Hermine O. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258.
[2] Sylvie Bonvalot , Alain Moussy , Jean-Pierre Kinet , et al. Phase II study of oral masitinib mesilate in imatinib-naïve patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). European Journal of Cancer. 2010(46): 1344-1351
Average Rating: 5 (Based on Reviews and 11 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




