MCC950 (CP-456773) (Synonyms: CP 456,773) |
| Catalog No.GC31644 |
MCC950 (CP-456773) (CP-456773; CRID3) is a potent and selective NLRP3 inhibitor with IC50s of 7.5 and 8.1 nM in BMDMs and HMDMs, respectively.
Products are for research use only. Not for human use. We do not sell to patients.
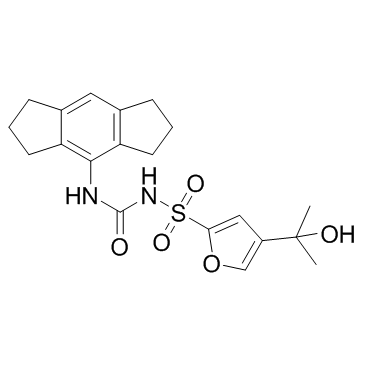
Cas No.: 210826-40-7
Sample solution is provided at 25 µL, 10mM.
MCC950 is a potent and selective NLRP3 inhibitor with IC50s of 7.5 and 8.1 nM in BMDMs and HMDMs, respectively.
MCC950 blocks canonical and non-canonical NLRP3 activation at nanomolar concentrations. MCC950 specifically inhibits NLRP3 but not AIM2, NLRC4 or NLRP1 activation. The effect of MCC950 on NLRP3 inflammasome activation is tested in mouse bone marrow derived macrophages (BMDM) and human monocyte derived macrophages (HMDM). The IC50 of MCC950 in BMDM is approximately 7.5 nM, while in HMDM it has a similar inhibitory capacity (IC50=8.1 nM). MCC950 also dose dependently inhibit IL-1β but not TNF-α secretion.MCC950 specifically blocks caspase-11-directed NLRP3 activation and IL-1β secretion upon stimulation of the non-canonical pathway. NLRC4-stimulated IL-1β and TNF-α secretion (as activated by Salmonella typhimurium) are not inhibited by MCC950 even at a concentration of 10 µM. MCC950 does not inhibit caspase-1 activation or IL-1β processing in response to S. typhimurium. The expression of pro-caspase-1 and pro-IL-1β in cell lysates is not substantially affected by MCC950 treatment[1].
MCC950 reduces Interleukin-1p (IL-1β) production and attenuates the severity of experimental autoimmune encephalomyelitis (EAE), a disease model of multiple sclerosis. Pre-treatment with MCC950 reduces serum concentrations of IL-1β and IL-6 while it does not considerably decrease the amount of TNF-α. Treatment of mice with MCC950 delays the onset and reduced the severity of EAE. Intracellular cytokine staining and FACS analysis of brain mononuclear cells from mice sacrificed on day 22 shows modestly reduced frequencies of IL-17 and IFN-γ producing CD3+ T cells in MCC950 treated mice in comparison with PBS-treated mice. IFN-γ and particularly IL-17 producing cell numbers are also reduced in both the CD4+ and γδ+ sub-populations of CD3+ T cells[1].
[1]. Coll RC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015 Mar;21(3):248-55. [2]. Gan W, et al. The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim Biophys Acta. 2017 Oct 3;1864(1):1-10. [3]. Zhang XY, et al. Propofol Does Not Reduce Pyroptosis of Enterocytes and Intestinal Epithelial Injury After Lipopolysaccharide Challenge. Dig Dis Sci. 2018 Jan;63(1):81-91. [4]. Qi Y, et al. NLRP3-dependent synaptic plasticity deficit in an Alzheimer’s disease amyloidosis model in vivo. Neurobiol Dis. 2018 Feb 23;114:24-30.
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




