MLN8237 (Alisertib) (Synonyms: Alisertib) |
| Catalog No.GC12690 |
An Aurora A kinase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
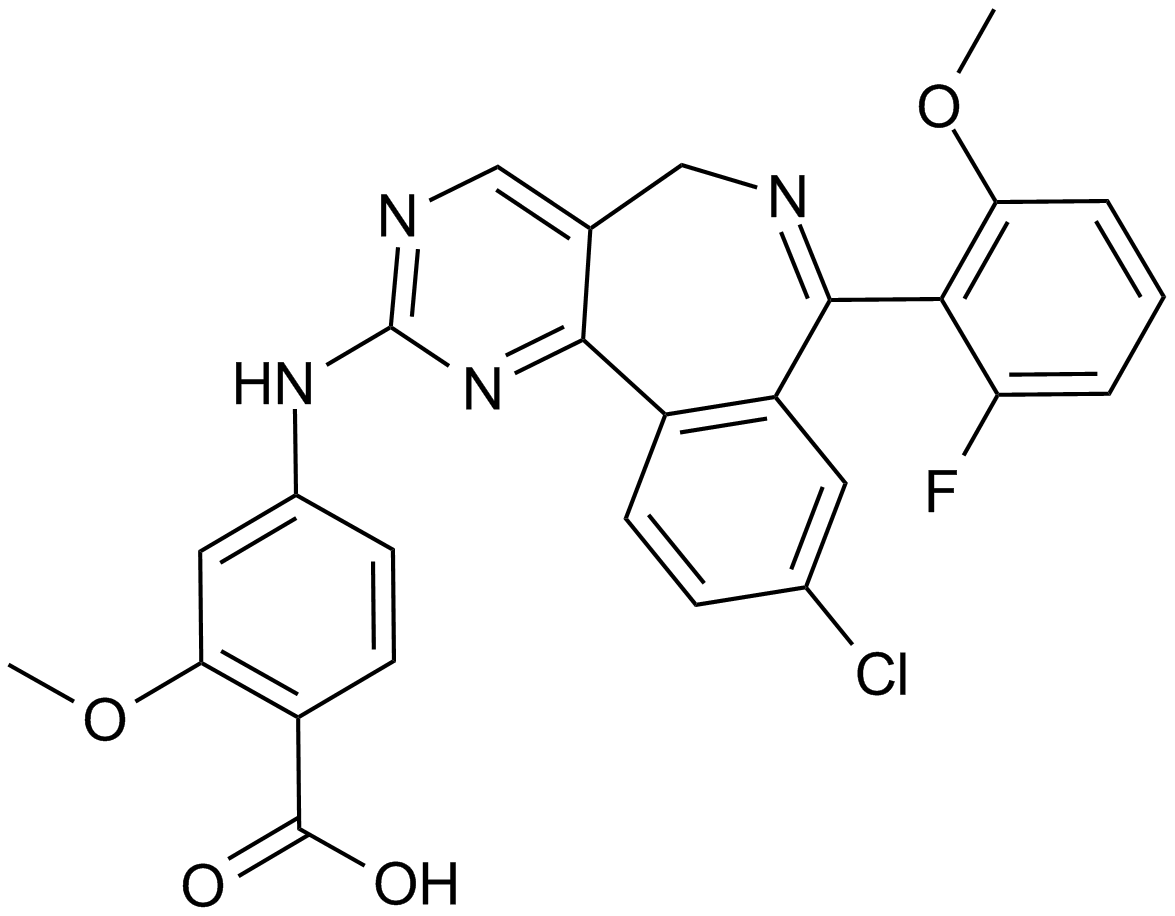
Cas No.: 1028486-01-2
Sample solution is provided at 25 µL, 10mM.
Alisertib (MLN8237), as an investigational, orally available, selective aurora A kinase inhibitor, is usually used for the treatment of solid tumors and hematologic malignancies.[1]
In vitro experiment it shown that treatment with 0.05 μM- 0.5 μM MLN8237 in the leukemia, medulloblastoma, and neuroblastoma cell lines inhibited their cell growth maximumly. However, with >1 μM approximately MLN8237, there was a paradoxical increase in apparent survival in all three lines, most pronounced for Daoy medulloblastoma cell line.[2] In vitro, MLN8237 is effective against both Ewing sarcoma and neuroblastoma cell lines with IC50 of 32 nM and 37 nM, respectively.[4] And alisertib has growth inhibition against U-2 OS cells and MG-63 cells with IC50 of 16.6 μM and 9.5 μM, respectively.[5] In addition, MLN8237 has aggressive inhibition against B-NHL cell proliferation with an IC50 of 10-50 nM and induced apoptosis with a dose- and time-dependent manner.[6]
In vivo, treatment with 30 mg/kg the combination of alisertib and lenvatinib intraperitoneally, every 3 days, for 4 consecutive weeks in BALB/c athymic nude mice, significantly enhanced the antiproliferative and proapoptotic activities, compared with single drugs alone.[3] In vivo efficacy test it indicated that mice were treated with 30 mg/kg MLN8237 for 21 days orally markedly reduced tumor burden and increased overall survival.[7]
References:
[1] Pusalkar S, et al. Biotransformation Pathways and Metabolite Profiles of Oral [14C]Alisertib (MLN8237), an Investigational Aurora A Kinase Inhibitor, in Patients with Advanced Solid Tumors. Drug Metab Dispos. 2020 Mar;48(3):217-229.
[2] Muscal JA, et al. Additive effects of vorinostat and MLN8237 in pediatric leukemia, medulloblastoma, and neuroblastoma cell lines. Invest New Drugs. 2013 Feb;31(1):39-45.
[3] Hao J, et al. Antitumor Effect of Lenvatinib Combined with Alisertib in Hepatocellular Carcinoma by Targeting the DNA Damage Pathway. Biomed Res Int. 2021 Jul 22;2021:6613439.
[4] Carol H, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011 Nov;68(5):1291-304.
[5] Niu NK, et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther. 2015 Mar 12;9:1555-84.
[6] Qi W, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011 Apr 1;81(7):881-90.
[7] G?rgün G, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010 Jun 24;115(25):5202-13.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




