Moguisteine (Synonyms: BBR 2173) |
| Catalog No.GC16326 |
novel peripheral non-narcotic antitussive drug
Products are for research use only. Not for human use. We do not sell to patients.
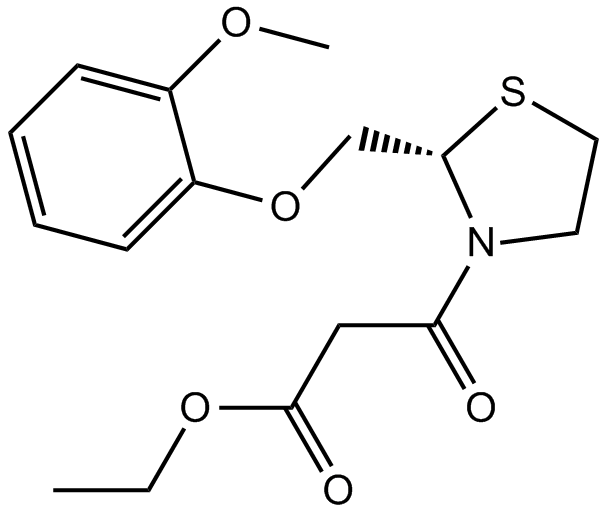
Cas No.: 119637-67-1
Sample solution is provided at 25 µL, 10mM.
Moguisteine(BBR-2173) is a novel peripheral non-narcotic antitussive drug.Target: OthersMoguisteine is a novel peripheral nonnarcotic antitussive agent that has proved to be as active as codeine in several experimental models of induced cough in guinea-pigs and dogs. It acts neither through the opiate receptors nor on the cough centre, and its action is possibly mediated by the interaction with rapidly adapting irritant receptors along the tracheobronchial tree. In controlled clinical trials, moguisteine has been shown to be safe and to effectively reduce cough associated with such respiratory disorders as acute upper respiratory tract infection, chronic bronchitis, pulmonary fibrosis and malignancies [1, 2].
References:
[1]. Gallico, L., et al., Moguisteine: a novel peripheral non-narcotic antitussive drug. Br J Pharmacol, 1994. 112(3): p. 795-800.
[2]. Gallico, L., et al., Effects of moguisteine, a peripheral nonnarcotic antitussive agent, on airway inflammation in guinea-pigs in vivo. Eur Respir J, 1996. 9(3): p. 478-85.
Average Rating: 5 (Based on Reviews and 26 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















