Moniliformin (sodium salt) |
| Catalog No.GC15592 |
induces mitotic arrest at the metaphase stage
Products are for research use only. Not for human use. We do not sell to patients.
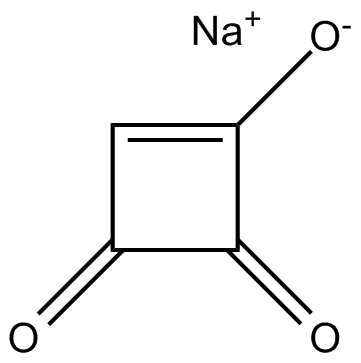
Cas No.: 71376-34-6
Sample solution is provided at 25 µL, 10mM.
Moniliformin induces mitotic arrest at the metaphase stage.
Mitosis is a part of the cell cycle when replicated chromosomes are separated into two new nuclei. The process of mitosis is divided into stages corresponding to the completion of one set of activities and the start of the next.
In vitro: Moniliformin, first isolated as a mycotoxin from Fusarium moniliforme, was found to be phytotoxic and arrests mitosis of maize root meristematic cells at the metaphase stage. The mitotic spindle could be disrupted by the treatment of moniliformin, but no direct effect on tubulin had been observed [1].
In vivo: A previous study was conducted on rat heart to in situ determine the myocardial toxicity of moniliformin, originally isolated from mouldy corn and soil samples in the Keshan disease prevalent area in China. Results showed that perfusion of moniliformin 10-7 mol/liter in isolated heart decreased myocardial contractile force by 52%. Intravenous injection of moniliformin at 1/6 and 1/4 LD50 could markedly inhibit cardiac hemodynamic variables associated with myocardial contractile function. Moreover, moniliformin was able to decrease +/- LV dP/dt max by 52%, and induce ventricular arrhythmia. These findings indicated that moniliformin was toxic to mammalian heart and might be an important factor relative to Keshan disease [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Duke, S. O. and Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins (Basel) 3(8), 1038-1064 (2011).
[2] Fan LL, Li J, Sun LH. Effect of moniliformin on myocardial contractility in rats. Biomed Environ Sci. 1991 Sep;4(3):290-4.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















