Mubritinib (TAK 165) (Synonyms: TAK-165) |
| Catalog No.GC10250 |
Mubritinib (TAK 165) (TAK-165) is a potent and selective EGFR2/HER2 inhibitor with an IC50 of 6 nM.
Products are for research use only. Not for human use. We do not sell to patients.
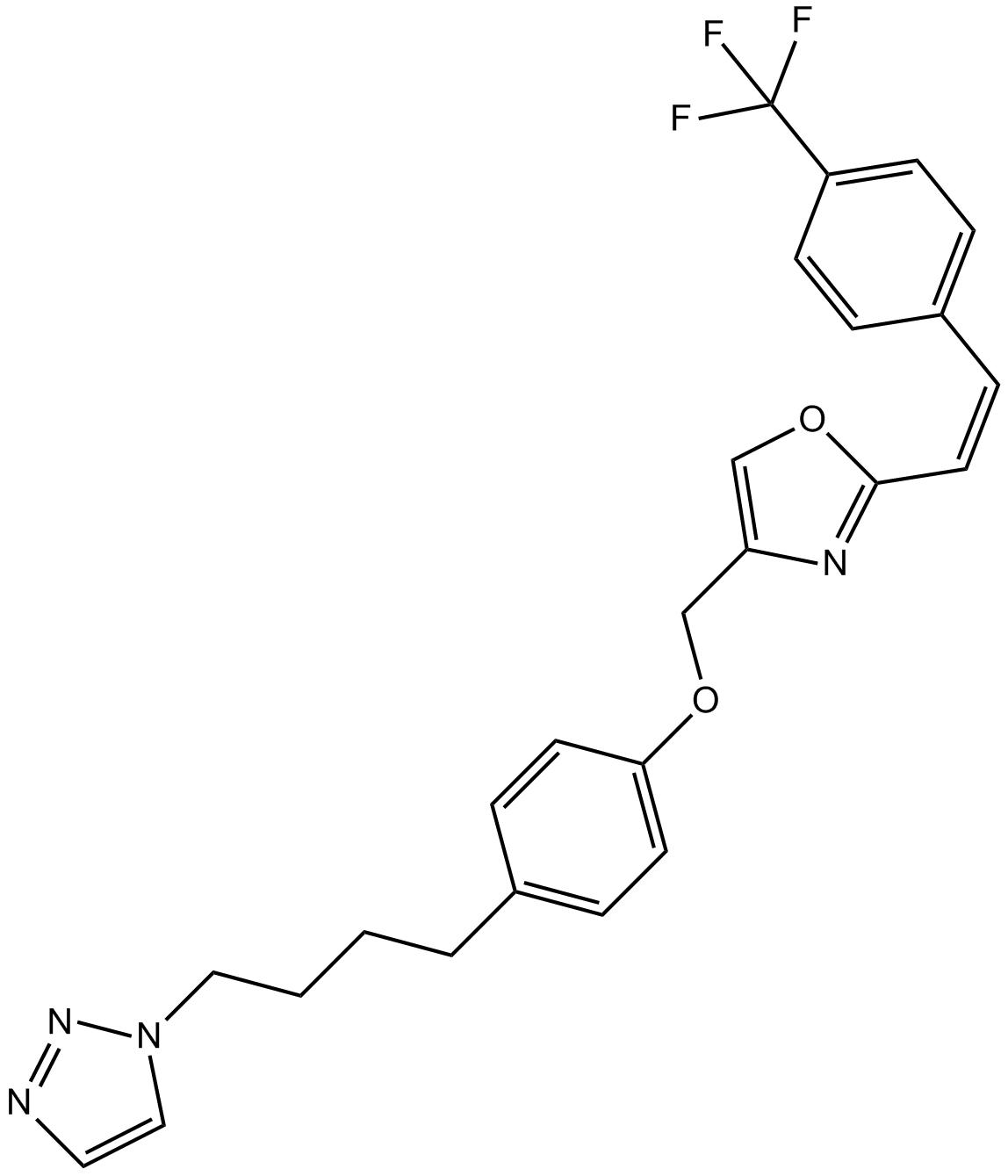
Cas No.: 366017-09-6
Sample solution is provided at 25 µL, 10mM.
Mubritinib (TAK-165) is a potent and selective EGFR2/HER2 inhibitor with an IC50 of 6 nM.
TAK-165 specifically inhibits HER2 tyrosine kinase with an IC50 6 nM and does not inhibit other types tyrosine kinase up to 25 000 nM. TAK-165 inhibits HER2 phosphorylation and its down-stream Akt and MAPK in HER2 strongly expressing cells (BT474 breast cancer cell line). TAK-165 sensitivity depends on HER2 levels of each cell line. Especially, BT474 cells which over-express HER2 strongly is highly sensitive (IC50=0.005 µM) and PC-3 cells which express HER2 very weakly is less sensitive (IC50=4.62 µM). But, HT1376 and ACHN cells that over-expressed EGFR showed high IC50 (IC50>25 µM)[1].
In the xenograft model, treatment with TAK-165 significantly inhibits growth of UMUC-3, ACHN, and LN-REC4. The antitumor effect after 14 days treatment are 22.9%, 26.0%, and 26.5% in UMUC3, ACHN and LN-REC4, respectively[1].
Reference:
[1]. Nagasawa J, et al. Novel HER2 selective tyrosine kinase inhibitor, TAK-165, inhibits bladder, kidney and androgen-independent prostate cancer in vitro and in vivo. Int J Urol. 2006 May;13(5):587-92.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















