Myristic Acid Alkyne (Synonyms: 13-alkyne Myristic Acid) |
| Catalog No.GC44258 |
Myristic Acid Alkyne is an alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs.
Products are for research use only. Not for human use. We do not sell to patients.
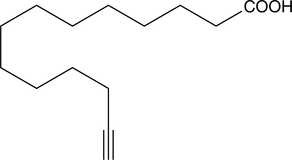
Cas No.: 82909-47-5
Sample solution is provided at 25 µL, 10mM.
Myristic acid is a 14-carbon saturated (14:0) fatty acid. In vivo, it is commonly added covalently to the N-terminus of proteins in a co-translational process termed N-myristoylation. [1] The sirtuin SIRT6 removes this acyl group from myristoylated TNF-α, enhancing secretion.[2] Myristic acid alkyne is a form of this myristic acid with an ω-terminal alkyne. Such terminal alkyne groups can be used in linking reactions, known as click chemistry, characterized by high dependability and specificity of azide-alkyne bioconjugation reactions. [3][4]Click chemistry has only recently been applied to the study of lipids.[2][5]
Reference:
[1]. Farazi, T.A., Waksman, G., and Gordon, J.I. The biology and enzymology of protein N-myristoylation. The Journal of Biological Chemisty 276(43), 39501-39504 (2001).
[2]. Jiang, H., Khan, S., Wang, Y., et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496, 110-113 (2013).
[3]. Kolb, H.C., and Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 8(24), 1128-1137 (2003).
[4]. Lutz, J.F., and Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne "click" chemistry. Adv. Drug Deliv. Rev. 60(9), 958-970 (2008).
[5]. Vila, A., Tallman, K.A., Jacobs, A.T., et al. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chemical Research in Toxicology 21(2), 432-444 (2008).
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















