N-acetyl-2-carboxy Benzenesulfonamide |
| Catalog No.GC16440 |
non-selective inhibitor of COX
Products are for research use only. Not for human use. We do not sell to patients.
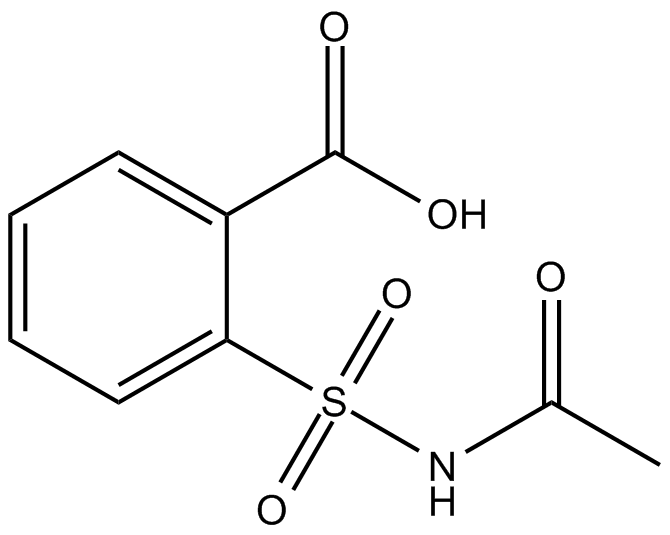
Cas No.: 849067-18-1
Sample solution is provided at 25 µL, 10mM.
IC50: 0.06 and 0.25 μM for COX-1 and COX-2, respectively
N-acetyl-2-carboxy Benzenesulfonamide is a non-selective inhibitor of COX.
Pharmaceutical inhibition of COX is able to provide relief from the symptoms of inflammation and pain. Nonsteroidal anti-inflammatory drugs, such as aspirin, exert its effect via inhibition of COX.
In vitro: Previous in-vitro COX-1/COX-2 inhibition studies showed that N-acetyl-2-carboxy benzenesulfonamide was a more potent inhibitor than aspirin, and like aspirin. Moreover, N-acetyl-2-carboxy benzenesulfonamide was found to be a nonselective COX-2 inhibitor. In addition, the molecular modeling (docking) study demonstrated that the SO2NHCOCH3 substituent present in N-acetyl-2-carboxy benzenesulfonamide, like the acetoxy substituent in aspirin, was suitably positioned to acetylate the Ser530 hydroxyl group in the COX-2 primary binding site [1].
In vivo: Animal study showed that N-acetyl-2-carboxy benzenesulfonamide and its C-4 2,4-difluorophenyl derivative had superior antiinflammatory activity (oral dosing) in a carrageenan-induced rat paw edema assay compared to aspirin. In addition, N-acetyl-2-carboxy benzenesulfonamide and its C-4 2,4-difluorophenyl derivative exhibited comparable analgesic activity to iflunisal, and superior analgesic activity compared to aspirin [1].
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] Chen, Q. H.,Rao, P.N.P., and Knaus, E.E. Design, synthesis, and biological evaluation of N-acetyl-2-carboxybenzenesulfonamides: A novel class of cyclooxygenase-2 (COX-2) inhibitors. Bioorganic & Medicinal Chemistry 13, 2459-2468 (2005).
Average Rating: 5 (Based on Reviews and 34 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















