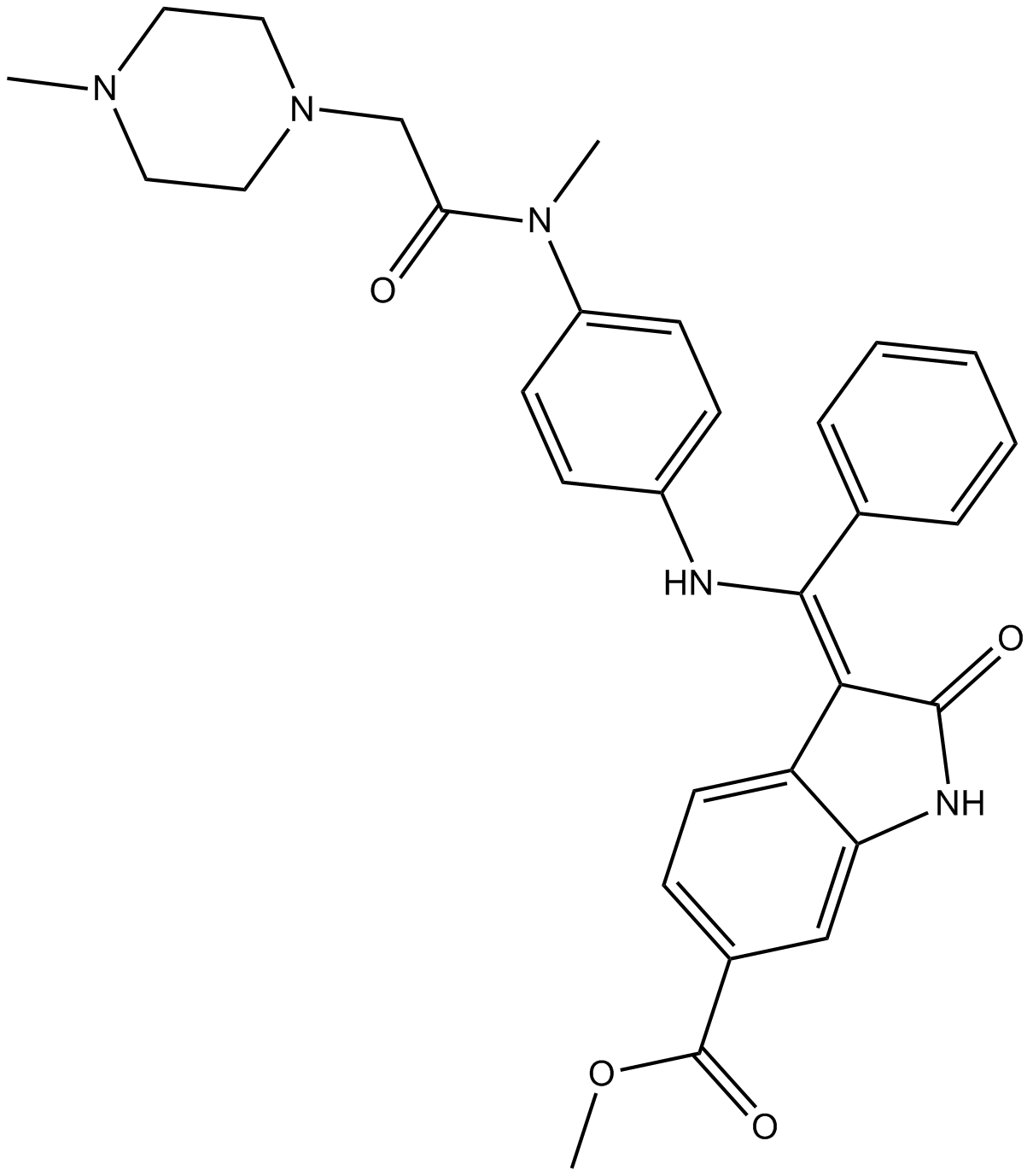
Nintedanib (BIBF 1120) (Synonyms: Vargatef) |
| Catalog No.GC11705 |
A VEGFR, FGFR, and PDGFR inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 656247-17-5
Sample solution is provided at 25 µL, 10mM.
Nintedanib (BIBF 1120) is an indolinone-derived oral active, triple angiokinase inhibitor of vascular endothelial growth factor receptor (VEGFR)1-3, fibroblast growth factor receptor (FGFR)1-3 and platelet-derived growth factor receptor (PDGFR)α/β1. It has shown potent antiangiogenic activity at nanomolar (IC50, 20-100 nmol/L) by blocking these receptor-mediated signaling pathways1,2. Nintedanib (BIBF 1120) is in clinical development for the treatment of idiopathic pulmonary fibrosis as these receptors have been shown to be potentially involved in the pathogenesis of pulmonary fibrosis3,4. As a novel angiogenesis inhibitor, it is also being widely evaluated in different cancer models and has displayed significant anti-tumor activities by inhibiting tumor blood vessel formation5-7.
To further evaluate its antitumor effects on multiple tumors, Nintedanib is currently entering several clinical trials, including non-small cell lung cancer8, ovarian cancer6, colorectal cancer7, hepatocellular carcinoma9 and many other solid tumors. In addition, the possibilities of combining Nintedanib therapy with other treatments such as docetaxel10 and afatinib 11are being tested in different tumor models. The most common drug-related adverse events in patients were diarrhea, nausea, vomiting and lethargy7.
References:
[1]Hilberg, F. et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer research 68, 4774-4782, doi:10.1158/0008-5472.CAN-07-6307 (2008).[2]Roth, G. J. et al. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120). Journal of medicinal chemistry 52, 4466-4480, doi:10.1021/jm900431g (2009).[3]Wollin, L., Maillet, I., Quesniaux, V., Holweg, A. & Ryffel, B. Antifibrotic and Anti-inflammatory Activity of the Tyrosine Kinase Inhibitor Nintedanib in Experimental Models of Lung Fibrosis. The Journal of pharmacology and experimental therapeutics 349, 209-220, doi:10.1124/jpet.113.208223 (2014).[4]Antoniu, S. A. Nintedanib (BIBF 1120) for IPF: a tomorrow therapy? Multidisciplinary respiratory medicine 7, 41, doi:10.1186/2049-6958-7-41 (2012).[5]Santos, E. S., Gomez, J. E. & Raez, L. E. Targeting angiogenesis from multiple pathways simultaneously: BIBF 1120, an investigational novel triple angiokinase inhibitor. Investigational new drugs 30, 1261-1269, doi:10.1007/s10637-011-9644-2 (2012).[6]Wei, X. W., Zhang, Z. R. & Wei, Y. Q. Anti-angiogenic drugs currently in Phase II clinical trials for gynecological cancer treatment. Expert opinion on investigational drugs 22, 1181-1192, doi:10.1517/13543784.2013.812071 (2013).[7]Mross, K. et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 16, 311-319, doi:10.1158/1078-0432.CCR-09-0694 (2010).[8]Rolfo, C. et al. BIBF 1120/ nintedanib : a new triple angiokinase inhibitor-directed therapy in patients with non-small cell lung cancer. Expert opinion on investigational drugs 22, 1081-1088, doi:10.1517/13543784.2013.812630 (2013).[9]Tai, W. T. et al. Nintedanib (BIBF-1120) inhibits hepatocellular carcinoma growth independent of angiokinase activity. Journal of hepatology, doi:10.1016/j.jhep.2014.03.017 (2014).[10]Reck, M. et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. The lancet oncology 15, 143-155, doi:10.1016/S1470-2045(13)70586-2 (2014).[11]Bouche, O. et al. Phase II trial of weekly alternating sequential BIBF 1120 and afatinib for advanced colorectal cancer. Anticancer research 31, 2271-2281 (2011).
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















