Olaparib (AZD2281, Ku-0059436) (Synonyms: AZD 2281, Ku0059436) |
| Catalog No.GC17580 |
A PARP inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
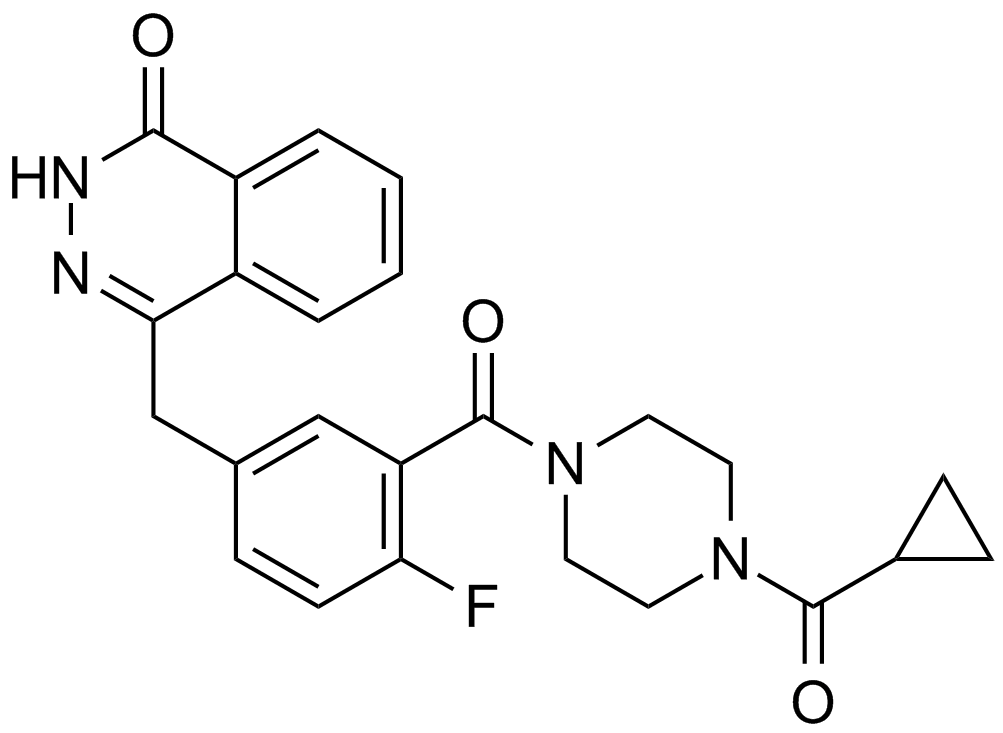
Cas No.: 763113-22-0
Sample solution is provided at 25 µL, 10mM.
Olaparib (AZD2281, Ku-0059436) is a potent and selective PARP inhibitor that specifically targets PARP1 and PARP2 (IC 50 = 5 nM and 1 nM, respectively). [1].It is an activator of autophagy and mitophagy.
Brca1-deficient cell lines were highly sensitive to PARP inhibition by Olaparib (AZD2281, Ku-0059436)[1]. Olaparib (AZD2281, Ku-0059436) enhances radiotherapy, not only by inhibiting DNA repair but also by changing tumor vascular hemodynamics in non-small cell lung carcinoma (NSCLC). In irradiated Calu-6 and A549 cells, Olaparib (AZD2281, Ku-0059436) enhanced the cytotoxic effects of radiation (sensitizer enhancement ratio at 10% survival = 1.5 and 1.3) and DNA double-strand breaks persisted for at least 24 hours after treatment[2]. PTEN-deficient endometrioid endometrial cancer cells are not responsive to PARP inhibitor Olaparib (AZD2281, Ku-0059436) alone, but instead show superior sensitivity to compound inhibition with PI3K inhibitor BKM120, as evidenced by reduced clonogenic cell growth and three-dimensional (3D) spheroid disintegration[4].
Considerable inhibition of tumor volumes as compared with that of the TMZ alone group was observed for the TMZ plus Olaparib (AZD2281, Ku-0059436)combination. This equated to over 80% tumor growth inhibition throughout the entire terminal phase of the study between TMZ treatment and the combination[1]. The PARP inhibitor AZD2281 (olaparib) showed synergetic effects with cisplatin in a dose-dependent manner. Combinatorial treatment with cisplatin and AZD2281 significantly inhibited xenografted tumor growth compared with single treatment of cisplatin or Olaparib (AZD2281, Ku-0059436)[3]. Treatment of tumor-bearing mice with AZD2281 inhibited tumor growth without signs of toxicity, resulting in strongly increased survival. Long-term treatment with Olaparib (AZD2281, Ku-0059436) in this model did result in the development of drug resistance[5]. DNA damage denoted by γH2AX foci was completely undetectable in primordial follicles of control animals but was observed in 10% of surviving primordial follicle oocytes in mice treated with Olaparib (AZD2281, Ku-0059436) alone[7]. When explored the possible combination of the PAPRi Olaparib (AZD2281, Ku-0059436) with EGFRvIII-targeted CAR (806-28Z CAR) T cells in immunocompetent mouse models of breast cancer.The administration of Olaparib (AZD2281, Ku-0059436) could significantly enhance the efficacy of 806-28Z CAR-T cells in vivo. Olaparib (AZD2281, Ku-0059436) could suppress myeloid-derived suppressor cell (MDSC) migration and promote the survival of CD8+ T cells in tumor tissue[6].
References:
[1]: Menear KA, Adcock C,et,al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem. 2008 Oct 23;51(20):6581-91. doi: 10.1021/jm8001263. Epub 2008 Sep 19. PMID: 18800822.
[2]: Senra JM, Telfer BA, et,al. Inhibition of PARP-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol Cancer Ther. 2011 Oct;10(10):1949-58. doi: 10.1158/1535-7163.MCT-11-0278. Epub 2011 Aug 8. PMID: 21825006; PMCID: PMC3192032.
[3]: Yasukawa M, Fujihara H, et,al. Synergetic Effects of PARP Inhibitor AZD2281 and Cisplatin in Oral Squamous Cell Carcinoma in Vitro and in Vivo. Int J Mol Sci. 2016 Feb 24;17(3):272. doi: 10.3390/ijms17030272. PMID: 26927065; PMCID: PMC4813136.
[4]: Bian X, Gao J, et,al. PTEN deficiency sensitizes endometrioid endometrial cancer to compound PARP-PI3K inhibition but not PARP inhibition as monotherapy. Oncogene. 2018 Jan 18;37(3):341-351. doi: 10.1038/onc.2017.326. Epub 2017 Sep 25. PMID: 28945226; PMCID: PMC5799770.
[5]: Rottenberg S, Jaspers JE, et,al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17079-84. doi: 10.1073/pnas.0806092105. Epub 2008 Oct 29. PMID: 18971340; PMCID: PMC2579381.
[6]: Sun R, Luo H, et,al.Olaparib Suppresses MDSC Recruitment via SDF1α/CXCR4 Axis to Improve the Anti-tumor Efficacy of CAR-T Cells on Breast Cancer in Mice. Mol Ther. 2021 Jan 6;29(1):60-74. doi: 10.1016/j.ymthe.2020.09.034. Epub 2020 Sep 26. PMID: 33010818; PMCID: PMC7791086.
[7]: Winship AL, Griffiths M, et,al. The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: implications for fertility preservation. Hum Reprod. 2020 Aug 1;35(8):1864-1874. doi: 10.1093/humrep/deaa128. PMID: 32604417.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




