Oxidopamine hydrochloride (6-Hydroxydopamine hydrochloride) |
| Catalog No.GC30871 |
Oxidopamine (6-OHDA) hydrochloride is an antagonist of the neurotransmitter dopamine.
Products are for research use only. Not for human use. We do not sell to patients.
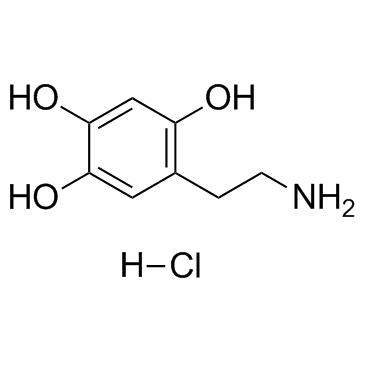
Cas No.: 28094-15-7
Sample solution is provided at 25 µL, 10mM.
Oxidopamine hydrochloride is a neurotoxic synthetic organic compound, selectively destroys dopaminergic and noradrenergic neurons in the brain.
Review for Oxidopamine hydrochloride (6-Hydroxydopamine hydrochloride)
Average Rating: 5 (Based on Reviews and 10 reference(s) in Google Scholar.)
Review for Oxidopamine hydrochloride (6-Hydroxydopamine hydrochloride)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















