Peptides
Products for Peptides
- Cat.No. Product Name Information
-
GC33788
Adrenocorticotropic Hormone (ACTH) (18-39), human (CLIP (human))
Adrenocorticotropic Hormone (ACTH) (18-39), human (CLIP (human)) is a corticotropinlike intermediate lobe peptide, which is produced in the melanotrophs of the intermediate lobe of the pituitary.

-
GC65059
Adrenocorticotropic Hormone (ACTH) (18-39), human TFA
Adrenocorticotropic Hormone (ACTH) (18-39), human TFA is a corticotropinlike intermediate lobe peptide, which is is produced in the melanotrophs of the intermediate lobe of the pituitary.
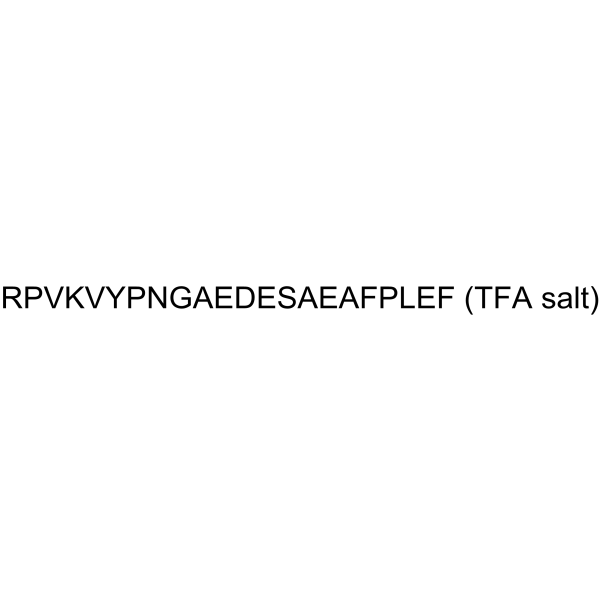
-
GC33790
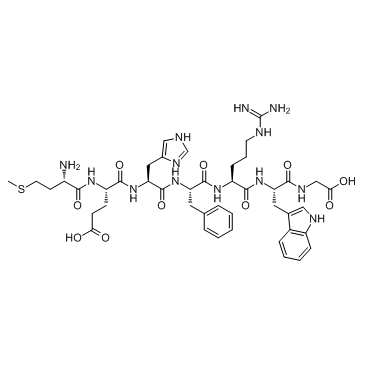
Adrenocorticotropic Hormone (ACTH) (4-10), human
Adrenocorticotropic Hormone (ACTH) (4-10), human is a melanocortin 4 (MC4R) receptor agonist.
-
GC42737
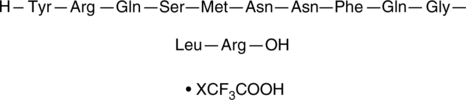
Adrenomedullin (1-12) (human) (trifluoroacetate salt)
Adrenomedullin (1-12) is an N-terminal fragment of adrenomedullin (1-52).
-
GC34230
Adrenomedullin (1-50), rat
Adrenomedullin (1-50), rat is a 50 amino acid peptide, which induces a selective arterial vasodilation via activation of CGRP1 receptor.

-
GC35256
Adrenomedullin (11-50), rat
Adrenomedullin (11-50), rat is the C-terminal fragment (11-50) of rat adrenomedullin.

-
GC42738
Adrenomedullin (13-52) (human) (trifluoroacetate salt)
Adrenomedullin (13-52) is a truncated form of adrenomedullin (1-52).

-
GC35257
Adrenomedullin (16-31), human
Adrenomedullin (16-31), human is amino acid residues 16-31 fragment of human adrenomedullin (hADM).

-
GC42741
Adrenomedullin (22-52) (human) (trifluoroacetate salt)
Adrenomedullin (22-52) is a C-terminal fragment of adrenomedullin (1-52).

-
GC33955
Adrenomedullin (AM) (1-52), human
Adrenomedullin (AM) (1-52), human is a 52-amino acid peptide, which affects cell proliferation and angiogenesis in cancer.

-
GC32622
Adrenomedullin (AM) (13-52), human
Adrenomedullin (AM) (13-52), human is a 40 amino acid peptide, which acts as an endothelium-dependent vasodilator agent.

-
GC32615
Adrenomedullin (AM) (22-52), human (22-52-Adrenomedullin (human))
Adrenomedullin (AM) (22-52), human (22-52-Adrenomedullin (human)), an NH2 terminal truncated adrenomedullin analogue, is an adrenomedullin receptor antagonist, and also antagonizes the calcitonin generelated peptide (CGRP) receptor in the hindlimb vascular bed of the cat.

-
GC11039
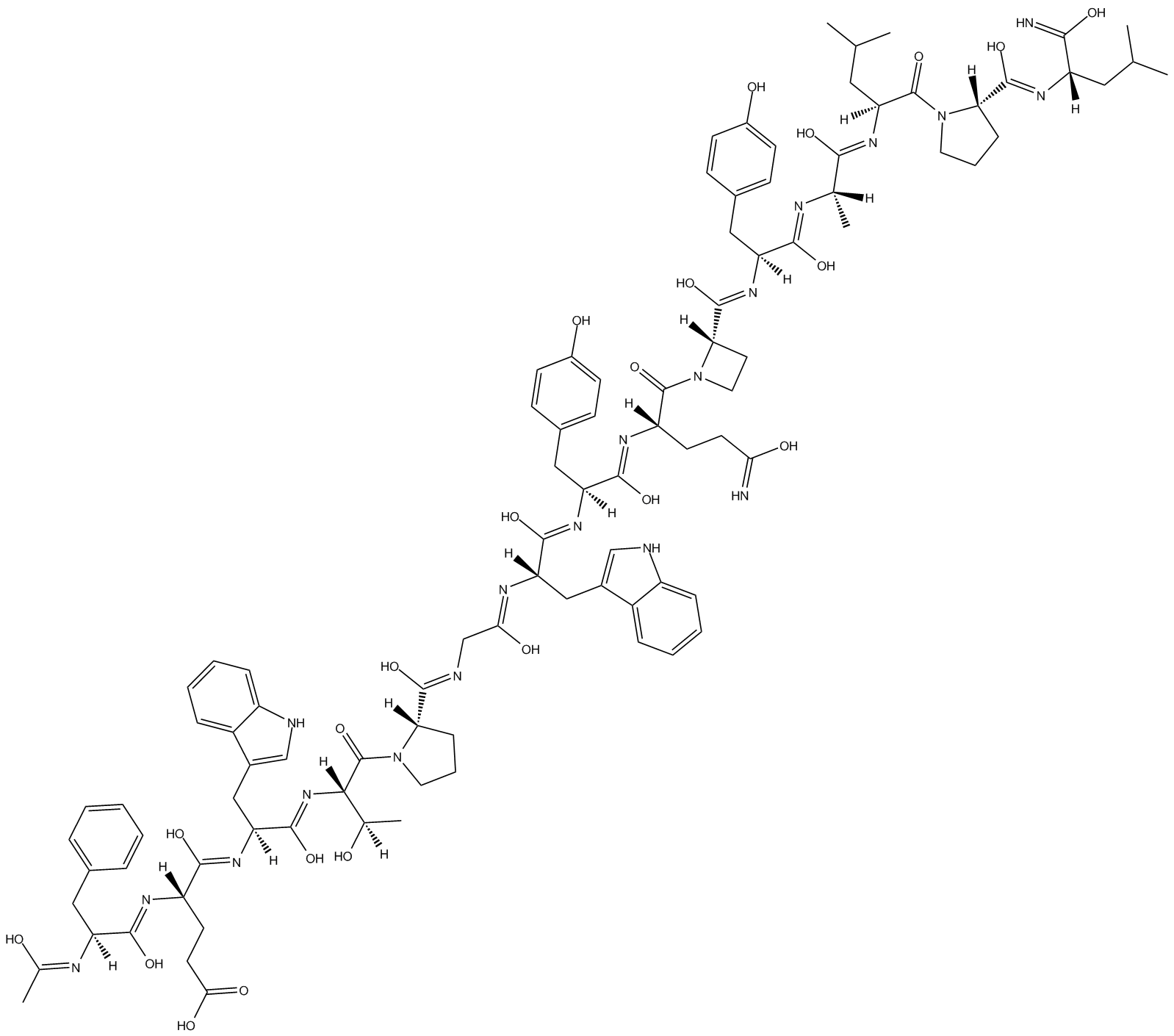
AF 12198
Type I IL-1 receptor antagonist
-
GC35264
AGA-(C8R) HNG17, Humanin derivative
AGA-(C8R) HNG17, Humanin derivative is a potent humanin (HN) derivative.
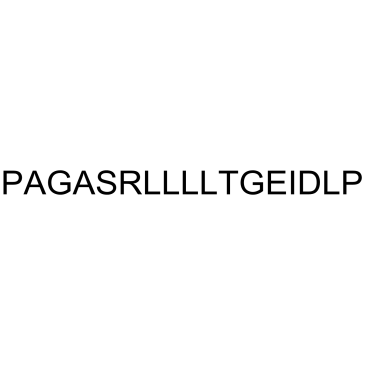
-
GC61526
AGA-(C8R) HNG17, humanin derivative TFA
AGA-(C8R) HNG17, humanin derivative TFA is a potent humanin (HN) derivative.

-
GC14867
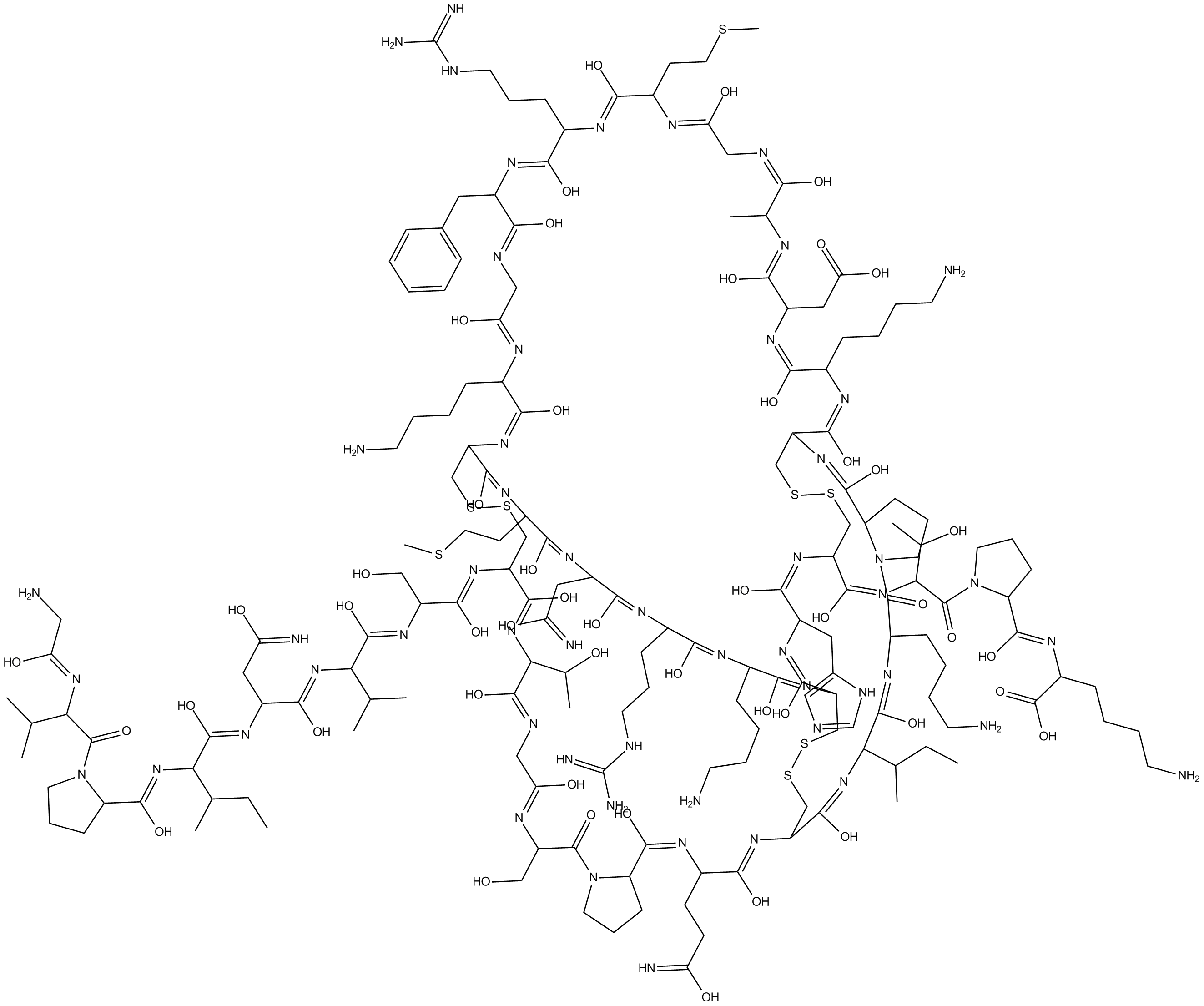
Agitoxin 2
Shaker K+ channel blocker, potent
-
GC15822
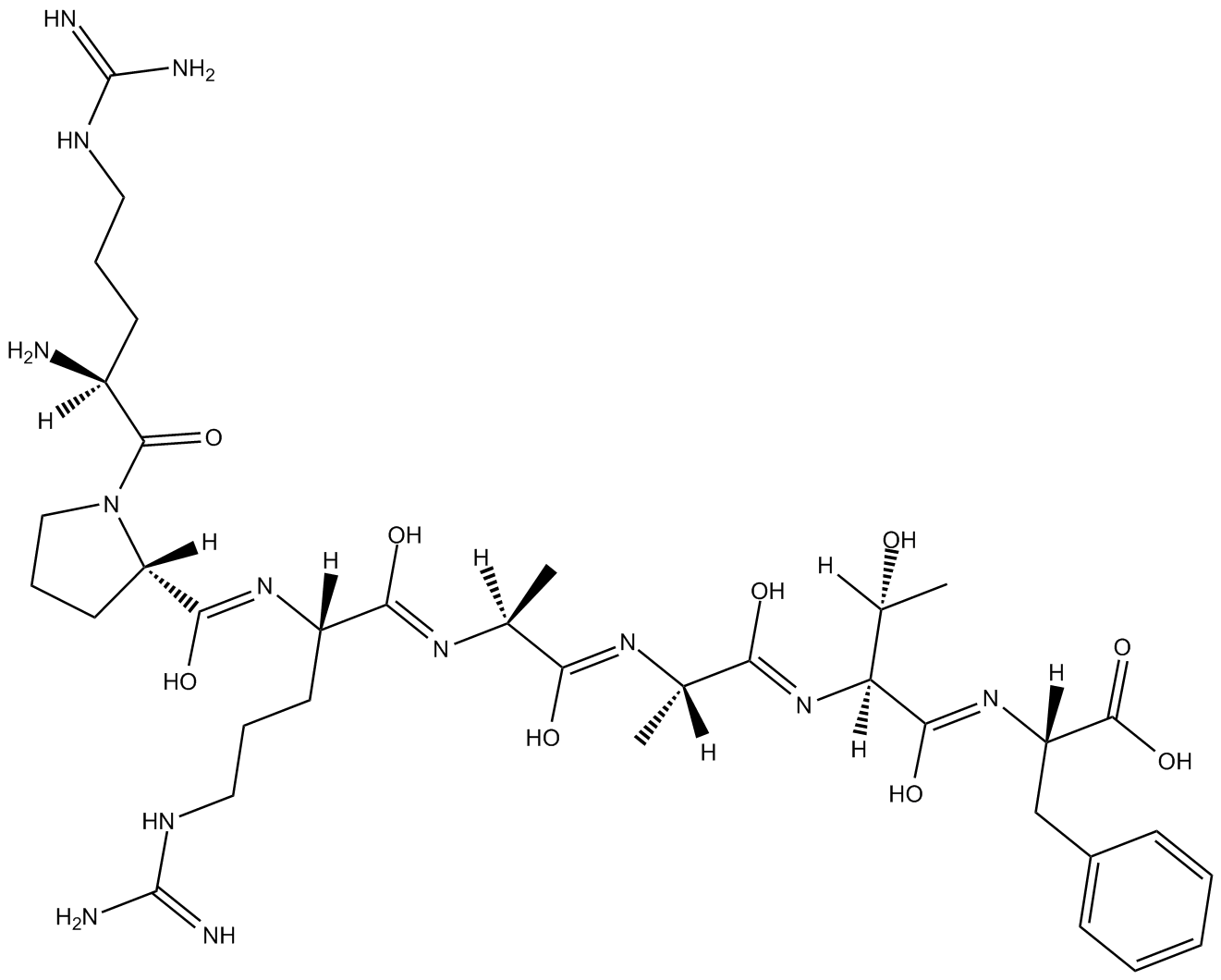
Akt/SKG Substrate Peptide
substrate for Akt/PKB
-
GC35282
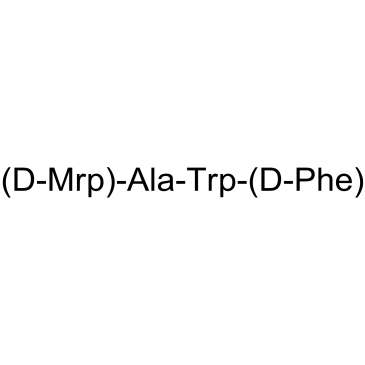
Alexamorelin Met 1
-
GC19584
Alkaline Phosphatase
10-50units/mg protein(37℃,pH 9.8)

-
GC35291
Allatostatin II
Allatostatin II is a decapeptid.

-
GC35292
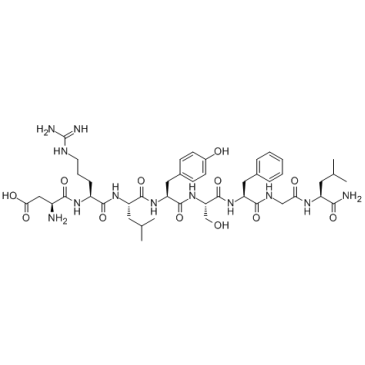
Allatostatin IV
Allatostatin IV is an octapeptide.
-
GC30940
Allergen Gal d 4 (46-61), chicken (Lysozyme C (46-61) (chicken))
Allergen Gal d 4 (46-61), chicken (Lysozyme C (46-61) (chicken)) is a hen egg white lysozyme peptide.

-
GC35304
Alpha 1(I) Collagen (614-639), human
Alpha 1(I) Collagen (614-639), human is a peptide fragment of alpha-1 type I collagen.
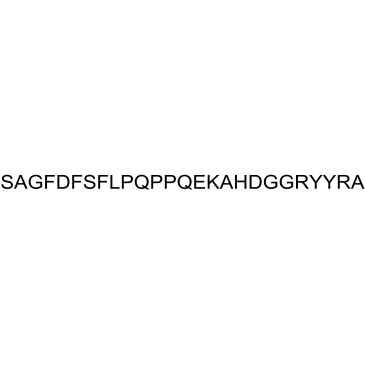
-
GC32208
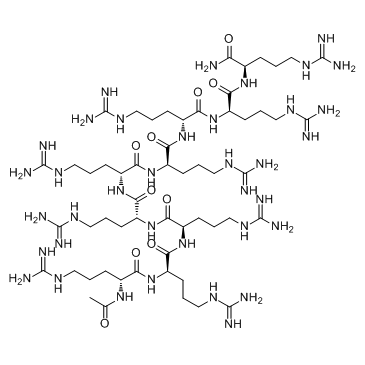
ALX 40-4C
ALX 40-4C is a small peptide inhibitor of the chemokine receptor CXCR4, inhibits SDF-1 from binding CXCR4 with a Ki of 1 μM, and suppresses the replication of X4 strains of HIV-1; ALX 40-4C Trifluoroacetate also acts as an antagonist of the APJ receptor, with an IC50 of 2.9 μM.
-
GC34386
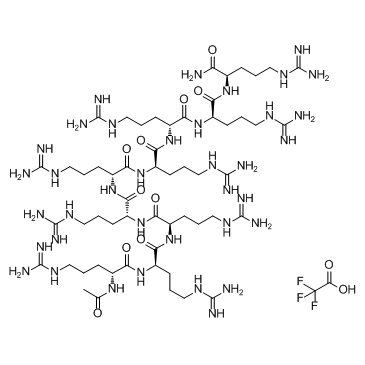
ALX 40-4C Trifluoroacetate
ALX 40-4C Trifluoroacetate is a small peptide inhibitor of the chemokine receptor CXCR4, inhibits SDF-1 from binding CXCR4 with a Ki of 1 μM, and suppresses the replication of X4 strains of HIV-1; ALX 40-4C Trifluoroacetate also acts as an antagonist of the APJ receptor, with an IC50 of 2.9 μM.
-
GC49262
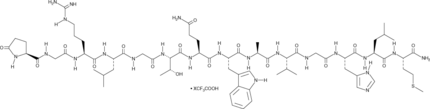
Alytesin (trifluoroacetate salt)
A neuropeptide with diverse biological activities
-
GC30514
AMARA peptide
AMARA peptide is a substrate for SIK and AMPK.

-
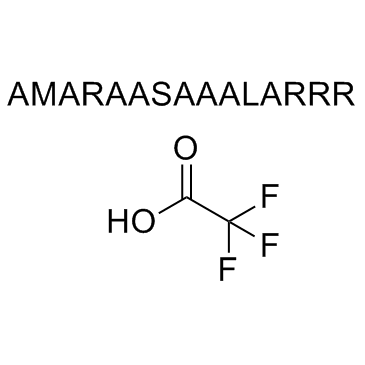
GC34224
AMARA peptide TFA
AMARA peptide (TFA) is a substrate for salt-inducible kinase (SIK) and adenosine monophosphate activated protein kinase (AMPK).
-
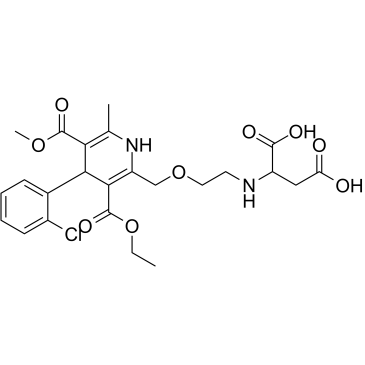
GC35324
Amlodipine aspartic acid impurity
Amlodipine aspartic acid impurity is the impurity of Amlodipine aspartic acid.
-
GC16513
Amylin
Amylin, a 37-amino acid polypeptide, is a pancreatic hormone cosecreted with insulin that exerts unique roles in metabolism and glucose homeostasis.

-
GA20710
Amylin (8-37) (human)
Human IAPP (8-37), ATQRLANFLVHSSNNFGAILSSTNVGSNTY-amide, readily forms fibrils in vitro.

-
GC35331
Amylin (8-37), rat
Amylin (8-37), rat is a truncated analog of native Amylin that selectively inhibits insulin-related glucose uptake and glycogen deposition in muscle tissue.

-
GA24066
Amylin (free acid) (human)

-
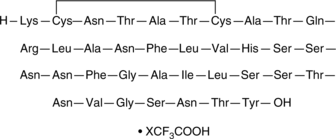
GC42796
Amylin (human) (trifluoroacetate salt)
Amylin is a 37-residue peptide hormone secreted from pancreatic β-cells that reduces food intake, decreases glucagon secretion, slows gastric emptying, and increases satiety.
-
GC35332
Amylin (IAPP), feline
Amylin (IAPP), feline is a 37-amino acid polypeptide from feline.

-
GC60581
Amylin (IAPP), feline TFA
Amylin (IAPP), feline TFA is a 37-amino acid polypeptide from feline.

-
GC35333
Amylin, amide, rat
Amylin, amide, rat is a potent and high affinity ligand of Amylin receptor AMY1 and AMY3 receptors and variably of AMY2 receptors; binding studies are generally used for the latter receptor.

-
GC35334
Amyloid β Peptide (42-1)(human)
Amyloid β Peptide (42-1)(human) is the inactive form of Amyloid β Peptide (1-42).

-
GC35335
Amyloid β-peptide (1-40) rat
Amyloid β-peptide (1-40) rat is a rat form of the amyloid β-peptide, which accumulates as an insoluble extracellular deposit around neurons, giving rise to the senile plaques associated with Alzheimer's disease (AD).

-
GA20721
Amyloid β-Protein (1-12)

-
GA20722
Amyloid β-Protein (1-14)
The N-terminal Aβ fragments Aβ1-14, Aβ1-15 (H-6368), and Aβ1-16 (H-2958) are elevated in cell media and in CSF in response to γ-secretase inhibitor treatment. The presence of these small peptides is consistent with a catabolic amyloid precursor protein cleavage pathway by β- followed by α-secretase. It has been shown that Aβ1-14, Aβ1-15, and Aβ1-16 increase dose-dependently in response to γ-secretase inhibitor treatment while Aβ1-42 levels are unchanged.

-
GA20724
Amyloid β-Protein (1-24)

-
GA20725
Amyloid β-Protein (1-37)
Amyloid β-Protein (1-37) correlates moderately with Mini-Mental State Examination (MMSE) scores in Alzheimer disease.

-
GA20726
Amyloid β-Protein (1-38)
Like Aβ (25-35) (H-1192), the Aβ fragment (1-38) destabilizes calcium homeostasis and renders human cortical neurones vulnerable to environmental insults.

-
GA20727
Amyloid β-Protein (1-39)
Small quantities of Aβ37, 38 and 39 can be detected in CSF together with Aβ40, the most abundant Aβ homolog, Aβ42, and N-terminally truncated amyloid peptides. The relative amounts depend on the variant of Alzheimer's disease. The C-terminally truncated amyloid peptides are also found in amyloid plaques.

-
GA20728
Amyloid β-Protein (1-40) (scrambled)

-
GA20729
Amyloid β-Protein (1-40) amide

-
GA24067
Amyloid β-Protein (1-40)-Lys(biotinyl) amide
For immobilization of Aβ40.

-
GA20733
Amyloid β-Protein (1-42)
Compared to the inner salt, the HCl salt of Aβ42 aggregates more readily at pH 7.4.

-
GA20730
Amyloid β-Protein (1-42) (HFIP-treated)
H-7442 was obtained by dissolving Amyloid β-Protein (1-42) (H-1368) in HFIP, aliquoting, and removing the solvent as described in the literature.

-
GA20731
Amyloid β-Protein (1-42) (scrambled)

-
GA24068
Amyloid β-Protein (1-42)-Lys(biotinyl) amide
For immobilization of Aβ42.

-
GA20736
Amyloid β-Protein (1-43)
Amyloid β-Protein (1-43) is more prone to aggregation and has higher toxic properties than the long-known Aβ1-42.

-
GA20737
Amyloid β-Protein (1-46)
Precursor of the secreted amyloid β-protein (1-40) and (1-42). The identification of amyloid-β-protein (1-46) led to the identification of a zeta-cleavage site between the known γ- and ε-cleavage sites within the transmembrane domain of amyloid-β precursor protein (APP).

-
GA20738
Amyloid β-Protein (1-6)
Experiments using sub-peptides of Aβ42 revealed that the epitope identified by the antibody A8, as described by Ying and coworkers, lies within the 1-6 region of Aβ. The antibody displays high affinity for soluble Aβ42 oligomers in the molecular weight range of 16.5-25 kDa, and detected target antigen in brain sections from senescence-accelerated SAMP 8 mice.

-
GA20739
Amyloid β-Protein (1-6) amide
Experiments using sub-peptides of Aβ42 revealed that the epitope identified by the antibody A8, as described by Ying and coworkers, lies within the 1-6 region of Aβ. The antibody displays high affinity for soluble Aβ42 oligomers in the molecular weight range of 16.5-25 kDa, and detected target antigen in brain sections from senescence-accelerated SAMP 8 mice. Amidated or acetylated and amidated forms of the sequence were used for example for quantitative structure retention relationships (QSRR) experiments. The latter could allow prediction of reversed-phase high-performance liquid chromatography (HPLC) retention of peptides, as reported by Kaliszan and coworkers.

-
GA20720
Amyloid β-Protein (10-35)
Amyloid β-protein (10-35), YEVHHQKLVFFAEDVGSNKGAIIGLM, was used as a truncated peptide model for the full-length amyloid β-proteins (1-40) and (1-42) in high-resolution structural studies. In contrast to the full-length amyloid β-proteins, amyloid β-protein (10-35) allowed the controlled and reproducible formation of homogeneous fibrils from aqueous solutions of defined pH, ionic strength and soluble peptide concentration necessary for high-resolution structural studies.

-
GA20723
Amyloid β-Protein (11-42)

-
GA20740
Amyloid β-Protein (16-20)
KLVFF corresponds to the minimum sequence binding the full-length amyloid β protein. The pentapeptide acts as a β-sheet breaker.

-
GA20741
Amyloid β-Protein (16-22)
Self-assembling Aβ sequence.

-
GA20742
Amyloid β-Protein (17-40)
Cleavage of APP by alpha- and gamma-secretase (i.e. the non-amyloidogenic pathway) yields p3 peptide, a mix of Aβ 17-40 and Aβ 17-42. p3 is a major constituent of diffuse plaques observed in AD brains and pre-amyloid plaques in people affected by Down syndrome.

-
GA24069
Amyloid β-Protein (17-42)
Cleavage of APP by alpha- and gamma secretase (i.

-
GA20744
Amyloid β-Protein (2-42)
Aβ 2-42 could be a biomarker for differentiating AD from other degenerative dementias, such as frontotemporal dementias (FTD). The peptide promotes phagocytosis by macrophages.

-
GA20743
Amyloid β-Protein (20-29)
FAEDVGSNKG.

-
GA20745
Amyloid β-Protein (25-35) amide
The amidation of amyloid β-protein (25-35) leads to a product in which the amyloidogenic capacity of amyloid β-protein (25-35) has been strongly reduced, while the neurotoxic activity was found to be independent of the aggregated state of the peptide.

-
GA20747
Amyloid β-Protein (3-40)

-
GA20748
Amyloid β-Protein (3-42)
The N-terminally truncated Aβ42 may be formed in increased amounts as AD progresses. Aβ 3-42 is the precursor of the Pyr-peptide. (Pyr³)-Aβ 3-42 positive plaques are resistant to age-dependent degradation likely due to their high stability and propensity to aggregate.

-
GA20746
Amyloid β-Protein (33-42)
GLMVGGVVIA, a partial sequence of β-amyloid protein which is used for raising antibodies against Aβ 1-42. Li et al. studied the aggregation behavior of this and other Aβ 1-42 C-terminal fragments.

-
GA20749
Amyloid β-Protein (35-25)
Reverse sequence of Aβ 25-35, inactive control.

-
GA20750
Amyloid β-Protein (36-38)

-
GA20751
Amyloid β-Protein (37-39)

-
GA20754
Amyloid β-Protein (4-42)
Aβ 4-42 could be one of the earliest and most prominent Aβ species deposited in AD brain. Sequencing of amyloid plaque cores showed that 64% of the isolated Aβ had a phenylalanine at its N-terminus, and indeed, IP/MS experiments identified Aβ 4-42 as a major Aβ species in AD patients. Additionally, Aβ 4-42 was found to be a component of cotton wool plaques in familial AD patients with the V261I PS1 mutation. Aβ 4-42 was discovered as well in amyloid deposits from vascular dementia and familial Danish dementia patients. These observations indicate that Aβ 4-42 may contribute to the development of multiple CNS diseases.

-
GA20753
Amyloid β-Protein (40-1)
Inactive control

-
GA20752
Amyloid β-Protein (40-1)
Reverse sequence of Aβ 1-40.

-
GA20755
Amyloid β-Protein (5-42)
Abeta 5-42 is produced from amyloid precursor protein by action of caspases. It is deposited in Alzheimer's disease brain as well, but less prone to aggregation.

-
GA20756
Amyloid β-Protein (6-20)

-
GA20718
Amyloid β/A4 Protein Precursor₇₇₀ (667-676)
The peptide substrate APP (667-676), SEVKMDAEFR, corresponds to the wild-type amyloid precursor protein (APP) β-secretase cleavage site. SEVKMDAEFR has been used for assaying β-secretase activity.

-
GA20719
Amyloid β/A4 Protein Precursor₇₇₀ (740-770)
Amyloid β/A4 Protein Precursor??? (740-770) corresponds to a C-terminal amyloid precursor protein (APP) fragment known as C31. This fragment is intracellularly generated by proteolytic cleavage of APP by caspases-8 and -9. C31 had a proapoptotic and a cytotoxic effect on neuronal cells and was shown to be present in brains of Alzheimer's disease (AD) patients. In cultured cells caspase cleavage of APP was induced by amyloid β-protein and the subsequent generation of C31 contributed to the apoptotic cell death associated with amyloid β-protein. Amyloid precursor binding protein BP1 (APP-BP1) a cell cycle protein which is increased in AD brain was demonstrated to bind to the C31 region of APP and to mediate APP-induced apoptosis.

-
GP10118
Amyloid Beta-Peptide (1-40) (human)
Amyloid β-Peptide (1-40) (human), (C194H295N53O58S1), a peptide with the sequence H2N-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA-OH, MW= 4329.8. Amyloid beta (Aβ or Abeta) is a peptide of 36–43 amino acids that is processed from the Amyloid precursor protein.
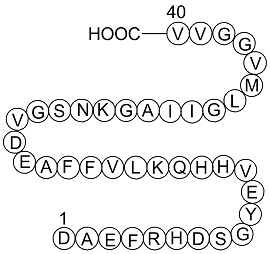
-
GP10049
Amyloid Beta-Peptide (12-28) (human)
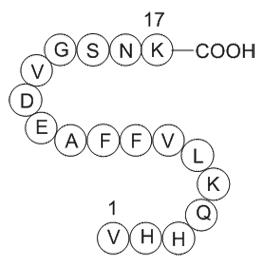
-
GP10082
Amyloid Beta-peptide (25-35) (human)
Amyloid beta-peptide (25-35) (human) is an fragment of Alzheimer's Amyloid beta peptide which has neurotoxic effects.
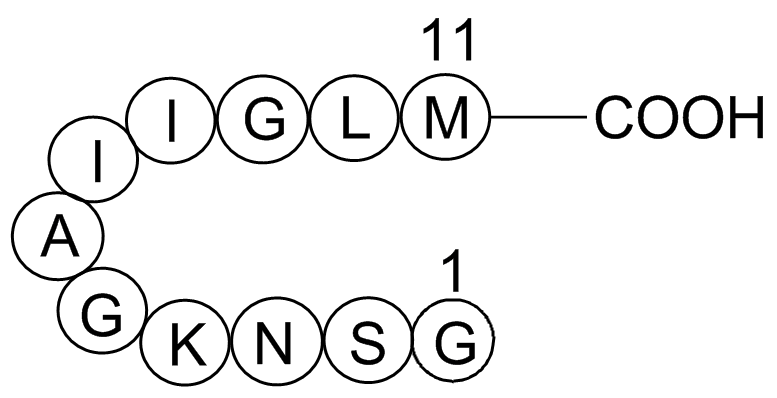
-
GC18339
Amyloid β-Peptide (1-42) human
Amyloid β-Peptide (1-42) human is a 42-amino acid peptide which plays a key role in the pathogenesis of Alzheimer disease.

-
GP10057
Amyloid β-Peptide (10-20) (human)
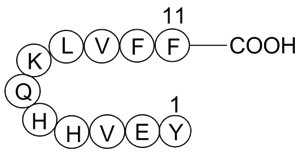
-
GP10094
Amyloid β-peptide (10-35), amide
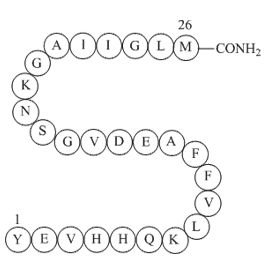
-
GP10097
Amyloid β-Protein (1-15)
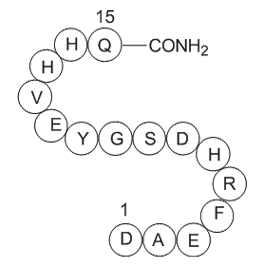
-
GC45382
Amyloid-β (1-28) Peptide (human) (trifluoroacetate salt)

-
GC42798
Amyloid-β (1-38) Peptide (trifluoroacetate salt)
Amyloid-β (1-38) (Aβ38) peptide is a fragment of the Aβ42 peptide.

-
GC46850
Amyloid-β (1-40) Peptide (human) (trifluoroacetate salt)
A neuropeptide with diverse biological activities

-
GC46851
Amyloid-β (1-42) Peptide (trifluoroacetate salt)
A 42-amino acid protein fragment of amyloid-β

-
GC42801
Amyloid-β (1-8) Peptide
Amyloid-β (1-8) is a wild-type control for the mutation-containing amyloid-β (1-8, A2V) peptide .

-
GC42802
Amyloid-β (1-8, A2V) Peptide
Amyloid-β (1-8, A2V) is a truncated form of amyloid-β (Aβ) that contains a valine to alanine substitution at position 2 of the Aβ numbering convention (Aβ A2V), which corresponds to position 673 of the amyloid precursor protein (APP) numbering convention (APP A673V).

-
GC42799
Amyloid-β (17-40) Peptide (human) (trifluoroacetate salt)
Amyloid-β (Aβ) (17-40) is a 24-residue fragment of the Aβ protein that is formed via post-translational processing of amyloid precursor protein (APP) by α- and γ-secretases.

-
GC42800
Amyloid-β (17-42) Peptide (human) (trifluoroacetate salt)
Amyloid-β (Aβ) (17-42) is a 26-residue fragment of the Aβ protein that is formed via post-translational processing of amyloid precursor protein (APP) by α- and γ-secretases.

-
GC40127
Amyloid-β (22-35) Peptide (trifluoroacetate salt)
Amyloid-β (Aβ) (22-35) is a 13-residue fragment of Aβ that corresponds to residues 693-705 of the human amyloid precursor protein (APP) full-length sequence.
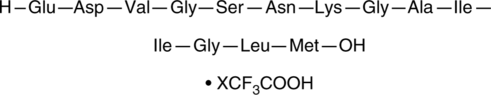
-
GC42803
Amyloid-β (25-35) Peptide (human) (trifluoroacetate salt)
Amyloid-β (25-35) (Aβ (25-35)) is an 11-residue fragment of the Aβ protein that retains the physical and biological characteristics of the full length peptide.
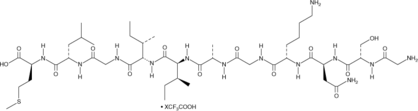
-
GC42804
Amyloid-β (40-1) Peptide (human) (trifluoroacetate salt)
Amyloid-β (40-1) peptide (Aβ (40-1)) is a peptide that contains the reverse sequence of Aβ (1-40) peptide.

-
GC40126
Amyloid-β Precursor Protein (96-110) Peptide (cyclized) (human) (trifluoroacetate salt)
Amyloid-β precursor protein (96-110) peptide (cyclized) is a synthetic peptide consisting of amino acids 96-110 of amyloid precursor peptide (APP) that is cyclized via a bridge between the cysteine residues at positions 3 and 10.

-
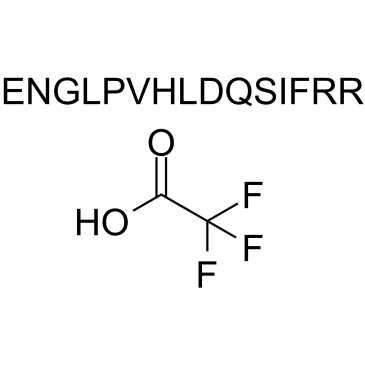
GC34471
Angiogenin (108-122) TFA
-
GC32607
Angiogenin 108-122
Angiogenin 108-122 is an angiogenin peptide.
-
GC61479
Angiopep-2 hydrochloride
Angiopep-2 hydrochloride is a brain peptide vector. The conjugation of anticancer agents with the Angiopep-2 peptide vector could increase their efficacy in the treatment of brain cancer.



