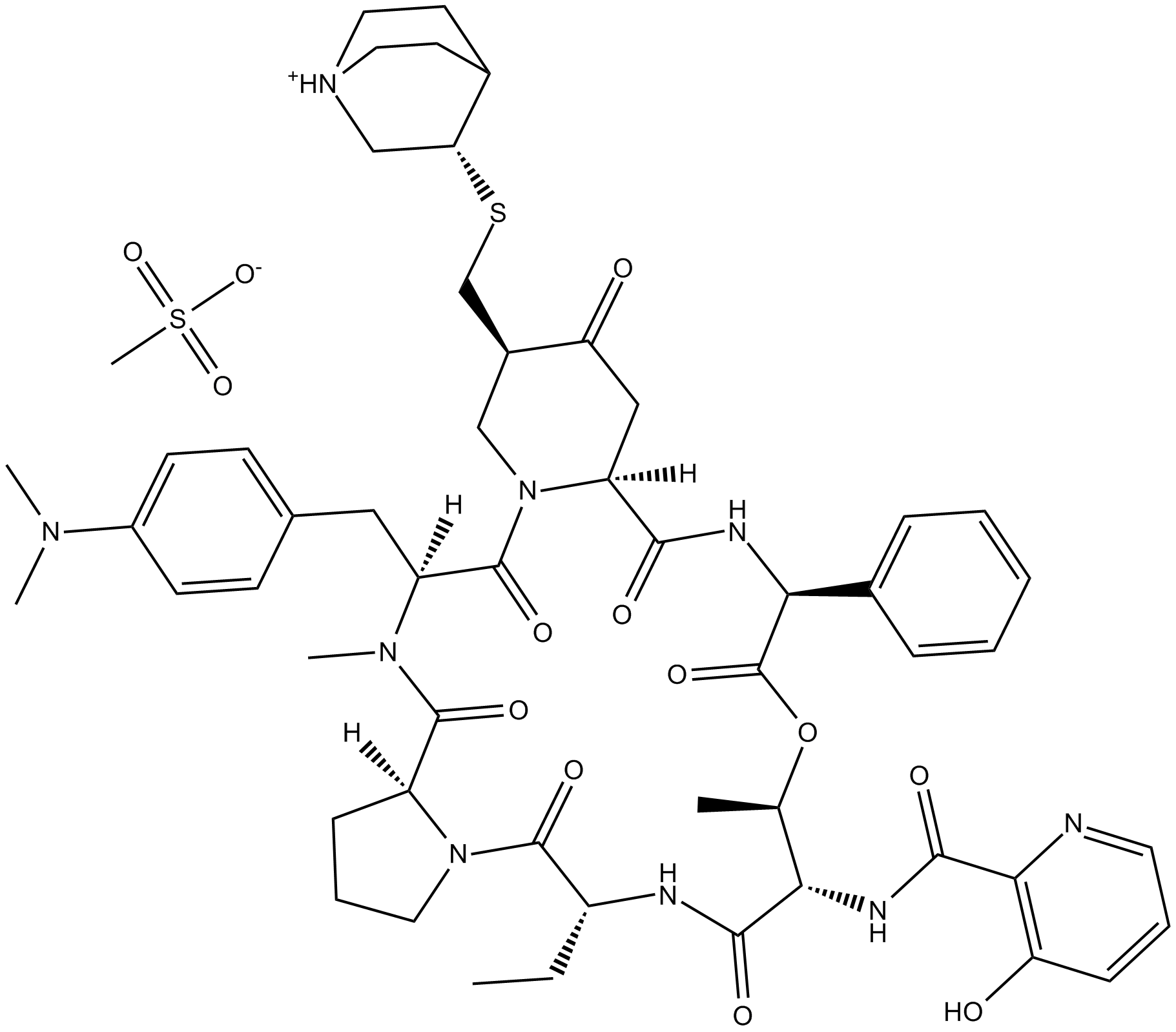
Quinupristin (mesylate) (Synonyms: RP 57669,Streptogramin B) |
| Catalog No.GC17579 |
Quinupristin (mesylate) is a streptogramin antibiotic.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 120138-50-3
Sample solution is provided at 25 µL, 10mM.
Quinupristin is a streptogramin antibiotic.
Streptogramins, a class of antibiotics, are effective in the treatment of vancomycin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus, which are two of the most rapidly growing strains of multidrug-resistant bacteria. Streptogramins fall into two groups: streptogramin A and streptogramin B.
In vitro: Quinupristin can bind to sequential sites located on the 50s subunit of the bacterial ribosome. Dalfopristin binding causes a conformational change in the ribosome that subsequently increases the binding of quinupristin. The combined actions of the two agents create a stable drug-ribosome complex causing inhibition of protein synthesis by prevention of peptide-chain formation, blockade of extrusion of newly formed peptide chains, and bacterial cell death [1].
In vivo: The combination of quinupristin-dalfopristin (Q-D) and gentamicin was tested against two strains of gentamicin- and dalfopristin-susceptible methicillin-resistant Staphylococcus aureus (MRSA). A rabbit endocarditis model simulated the pharmacokinetics achieved in humans receiving intravenous injections of Q-D and gentamicin. For the MLSB-susceptible strain, a 4-day regimen reduced mean bacterial titers (MBT) in vegetations from 8.5 ± 0.8 log CFU/g (control group) to 3.0 ± 0.9 (Q-D) and 2.6 ± 0.5 log CFU/g (Q-D plus gentamicin). For the strain constitutively resistant to MLSB, a 4-day regimen reduced MBT in vegetations from 8.7 ± 0.9 log CFU/g (control group) 5.2 ± 2.2 (Q-D) and and 5.1 ± 2.4 log CFU/g (Q-D plus gentamicin). The differences between control and treatment groups were significant for both strains, although there was no significant difference between treatment groups [2].
Clinical trial: The combination quinupristin/dalfopristin (Synercid) was brought to the market by in 1999. Synercid is clinically used to treat infections by staphylococci and by vancomycin-resistant Enterococcus faecium [3].
References:
[1] Allington D R, Rivey M R Quinupristid/Dalfopristin: A Therapeutic Review. Clinical Therapeutics .200 1, 23,(1): 24-44.
[2] Batard E, Jacqueline C, Boutoille D, Hamel A, Drugeon HB, Asseray N, Leclercq R, Caillon J, Potel G, Bugnon D. Combination of quinupristin-dalfopristin and gentamicin against methicillin-resistant Staphylococcus aureus: experimental rabbit endocarditis study. Antimicrob Agents Chemother. 2002 Jul;46(7):2174-8.
[3] https://en. wikipedia.org/wiki/Dalfopristin
Average Rating: 5 (Based on Reviews and 2 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















