Proteases
Proteases is a general term for a class of enzymes that hydrolyze protein peptide chains. According to the way they degrade polypeptides, they are divided into two categories: endopeptidases and telopeptidases. The former can cut the large molecular weight polypeptide chain from the middle to form prions and peptones with smaller molecular weights; the latter can be divided into carboxypeptidase and aminopeptidase, which respectively remove the peptide from the free carboxyl terminus or free amino terminus of the polypeptide one by one. Chain hydrolysis produces amino acids.
A general term for a class of enzymes that hydrolyze peptide bonds in proteins. According to the way they hydrolyze polypeptides, they can be divided into endopeptidases and exopeptidases. Endopeptidase cleaves the interior of the protein molecule to form smaller molecular weight peptones and peptones. Exopeptidase hydrolyzes peptide bonds one by one from the end of the free amino group or carboxyl group of protein molecules, and frees amino acids, the former is aminopeptidase and the latter is carboxypeptidase. Proteases can be classified into serine proteases, sulfhydryl proteases, metalloproteases and aspartic proteases according to their active centers and optimum pH. According to the optimum pH value of its reaction, it is divided into acidic protease, neutral protease and alkaline protease. The proteases used in industrial production are mainly endopeptidases.
Proteases are widely found in animal offal, plant stems and leaves, fruits and microorganisms. Microbial proteases are mainly produced by molds and bacteria, followed by yeast and actinomycetes.
Enzymes that catalyze the hydrolysis of proteins. There are many kinds, the important ones are pepsin, trypsin, cathepsin, papain and subtilisin. Proteases have strict selectivity for the reaction substrates they act on. A protease can only act on certain peptide bonds in protein molecules, such as the peptide bonds formed by the hydrolysis of basic amino acids catalyzed by trypsin. Proteases are widely distributed, mainly in the digestive tract of humans and animals, and are abundant in plants and microorganisms. Due to limited animal and plant resources, the industrial production of protease preparations is mainly prepared by fermentation of microorganisms such as Bacillus subtilis and Aspergillus terrestris.
Targets for Proteases
- Caspase(114)
- Aminopeptidase(24)
- ACE(74)
- Calpains(20)
- Carboxypeptidase(10)
- Cathepsin(81)
- DPP-4(31)
- Elastase(26)
- Gamma Secretase(67)
- HCV Protease(59)
- HSP(113)
- HIV Integrase(37)
- HIV Protease(47)
- MMP(228)
- NS3/4a protease(8)
- Serine Protease(18)
- Thrombin(58)
- Urokinase(4)
- Cysteine Protease(0)
- Other Proteases(18)
- Tyrosinases(47)
- 15-PGDH(1)
- Acetyl-CoA Carboxylase(13)
- Acyltransferase(59)
- Aldehyde Dehydrogenase (ALDH)(28)
- Aminoacyl-tRNA Synthetase(9)
- ATGL(1)
- Dipeptidyl Peptidase(56)
- Drug Metabolite(457)
- E1/E2/E3 Enzyme(90)
- Endogenous Metabolite(1636)
- FABP(30)
- Farnesyl Transferase(23)
- Glutaminase(14)
- Glutathione Peroxidase(14)
- Isocitrate Dehydrogenase (IDH)(28)
- Lactate Dehydrogenase(17)
- Lipoxygenase(234)
- Mitochondrial Metabolism(207)
- NEDD8-activating Enzyme(7)
- Neprilysin(12)
- PAI-1(13)
- Ser/Thr Protease(41)
- Tryptophan Hydroxylase(13)
- Xanthine Oxidase(18)
- MALT1(10)
- PCSK9(1)
Products for Proteases
- Cat.No. Product Name Information
-
GC49118
10-hydroxy Warfarin
A metabolite of (R)-warfarin
-
GC33800
10Z-Nonadecenoic acid
A monounsaturated fatty acid
-
GC40368
11(R)-HEPE
11(R)-HEPE is produced by the oxidation of EPA by 11(R)-LO.
-
GC40445
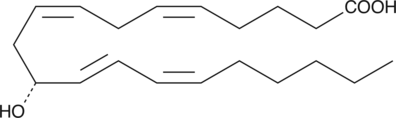
11(R)-HETE
11(R)-HETE is biosynthesized by 11(R)-LOs of the sea urchin, S.
-
GC39223
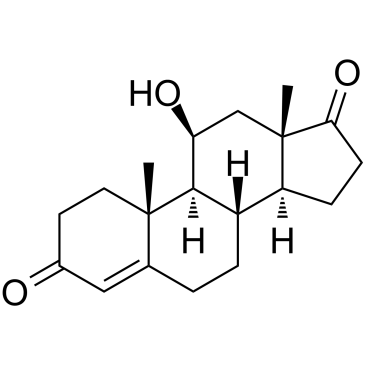
11-Beta-hydroxyandrostenedione
A non-androgenic steroid
-
GC40730
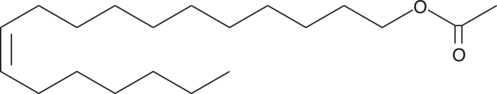
11-cis Vaccenyl Acetate
The mating and social behaviors of insects are largely orchestrated by a suite of volatile hydrocarbon pheromones.
-
GC40394
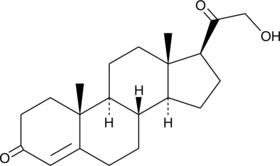
11-deoxy Corticosterone
11-deoxy Corticosterone is a steroid hormone produced by the adrenal gland that possesses mineralocorticoid activity and acts as an aldosterone precursor.
-
GC10821
11-keto-β-Boswellic Acid
11-Keto-beta-boswellic acid (11-Keto-β-boswellic acid) is a pentacyclic triterpenic acid of the oleogum resin from the bark of the Boswellia serrate tree, popularly known as Indian Frankincense. 11-Keto-beta-boswellic acid has the anti-inflammatory activity is primarily due to inhibit 5-lipoxygenase (5-LOX) and subsequent leukotriene and nuclear factor-kappa B (NF-κB) activation and tumor necrosis factor alpha generation production.

-
GC61538
11-Oxo etiocholanolone
11-Oxo etiocholanolone (11-Ketoetiocholanolone) is a metabolite of Etiocholanolone.
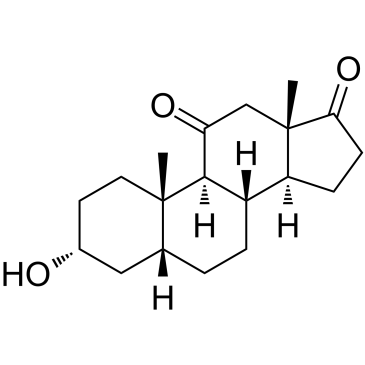
-
GC41144
11-trans Leukotriene C4
11-trans Leukotriene C4 (11-trans LTC4) is a C-11 double bond isomer of LTC4.
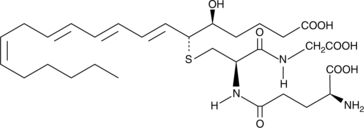
-
GC41147
11-trans Leukotriene D4
11-trans Leukotriene D4 (11-trans LTD4) is a C-11 double bond isomer of LTD4.
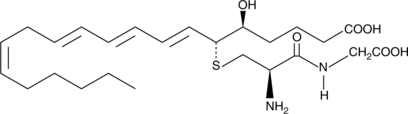
-
GC41149
11-trans Leukotriene E4
Slow isomerization of the C-11 double bond of LTE4 leads to the formation of 11-trans LTE4.
-
GC63796
116-9e
116-9e (MAL2-11B) is a Hsp70 co-chaperone DNAJA1 inhibitor.
-
GC34016
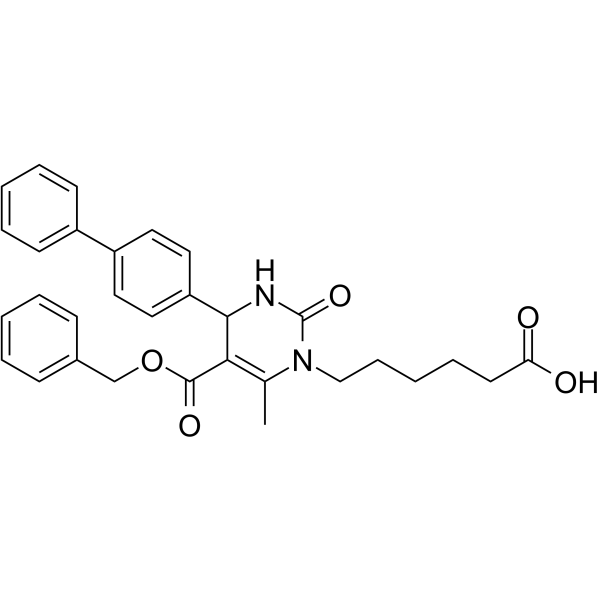
11beta-Hydroxyprogesterone (11β-Hydroxyprogesterone)
11beta-Hydroxyprogesterone (11β-Hydroxyprogesterone) is a potent inhibitors of 11β-Hydroxysteroid dehydrogenase; also activates human mineralocorticoid receptor in COS-7 cells with an ED50 of 10 nM.
-
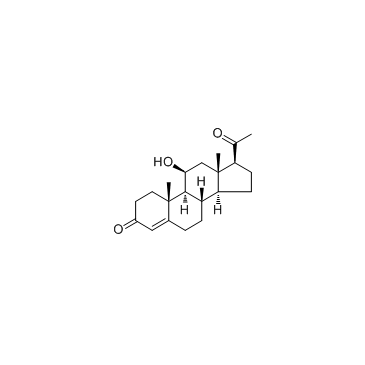
GC40447
12(R)-HETE
Biosynthesis of 12(R)-HETE in invertebrates is via lipoxygenation of arachidonic acid.
-
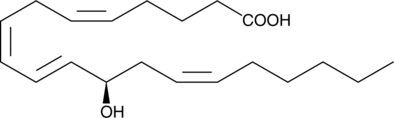
GC40371
12(S)-HEPE
12(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 12-LO.
-
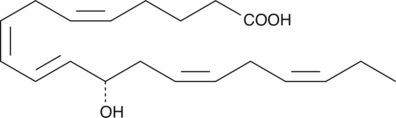
GC40448
12(S)-HETE
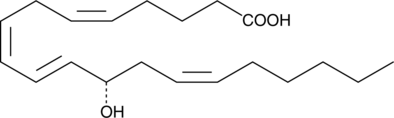
12(S)-HETE is the predominant lipoxygenase product of mammalian platelets.
-
GC41882
12(S)-HETrE
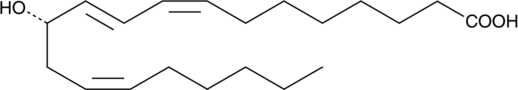
12(S)-HETrE is produced by 12-lipoxygenase oxidation of dihomo-γ-linolenic acid (DGLA).
-
GC41095
12(S)-HpEPE
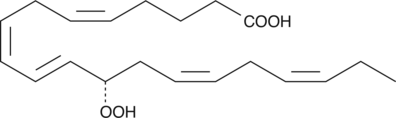
12(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 12-lipoxygenase on eicosapentaenoic acid.
-
GC41122
12(S)-HpETE
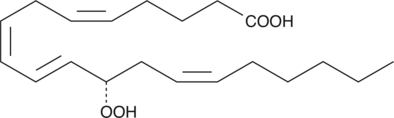
12(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of platelet or leukocyte 12-lipoxygenase (12-LO) on arachidonic acid.
-
GC41123
12-epi Leukotriene B4
Leukotriene B4 (LTB4) compounds are produced by both enzymatic and non-enzymatic processes.
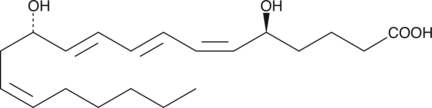
-
GC45962
12-hydroxy Lauric Acid
12-hydroxy Lauric Acid is an endogenous metabolite.
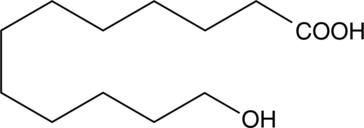
-
GC60443
12-Ketodeoxycholic acid
A metabolite of DCA
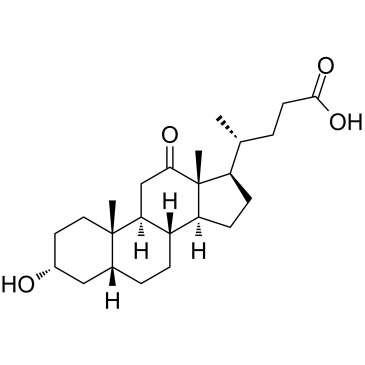
-
GC41096
12-oxo Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-LO pathway.
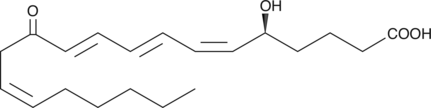
-
GC46418
12-oxo-13-HOME
An oxylipin
-
GC40372
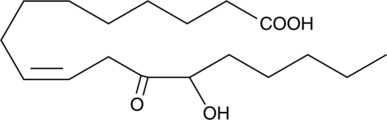
12-OxoETE
12-OxoETE is synthesized by human platelets and Aplysia nervous tissue after incubation with arachidonic acid.
-
GC19462
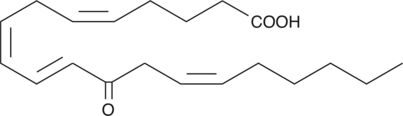
13(R)-HODE
13(R)-HODE is the opposite enantiomer of the 13(S)-HODE produced when linoleic acid is incubated with soybean lipoxygenase.
-
GC19463
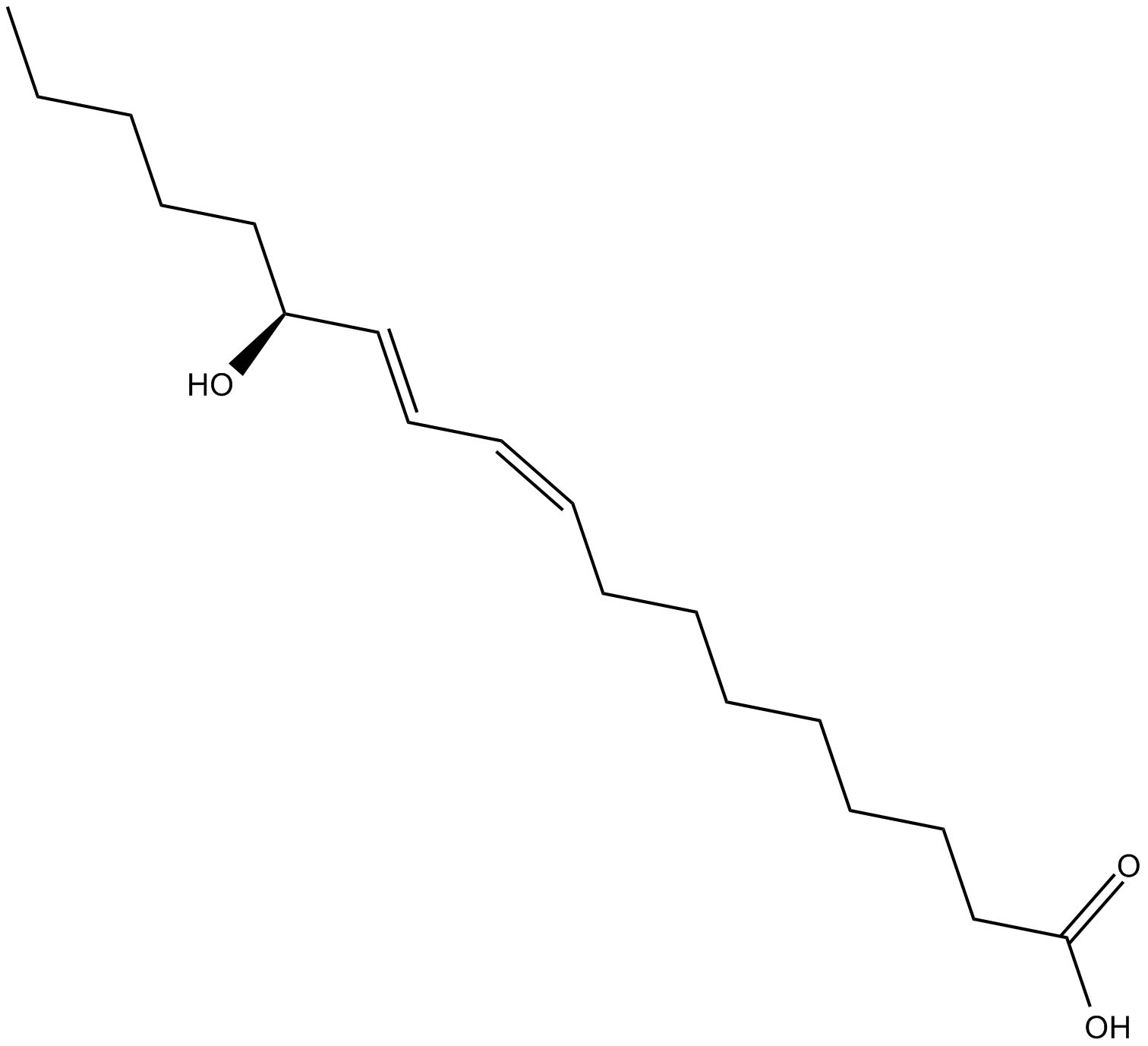
13(S)-HODE
13(S)-HODE (13(S)-HODE), the product of 15-lipoxygenase (15-LOX) metabolism of linoleic acid, functions as the endogenous ligand to activate PPARγ.
-
GC41220
13(S)-HODE methyl ester
13(S)-hydroxyoctadecadienoic acid (13(S)-HODE) is a 15-lipoxygenase metabolite of linoleic acid produced in endothelial cells, leukocytes, and tumor cells.
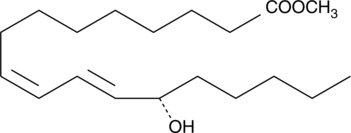
-
GC41896
13(S)-HODE-biotin
13(S)-HODE is the lipoxygenase metabolite of linoleic acid.
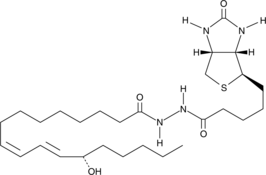
-
GC46420
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE
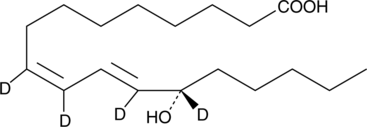
-
GC41897
13(S)-HOTrE
13(S)-HOTrE is the 15-lipoxygenase (15-LO) product of linolenic acid.
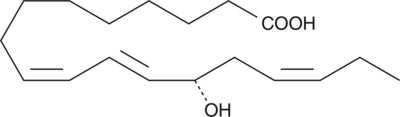
-
GC41898
13(S)-HOTrE(γ)
13(S)-HOTrE(γ) is the 15-LO product of γ-linolenic acid.
-
GC19474
13(S)-HpODE
13(S)-HpODE is produced by the oxidation of linoleic acid by lipoxygenase-1 (LO-1) in many plants including soybean, flaxseed, apples, and tea leaves,1,2 and by 15-LO in mammals.
-
GC41899
13(S)-HpOTrE
13(S)-HpOTrE is a monohydroperoxy polyunsaturated fatty acid produced in soybeans by the action of soybean LO-2 on esterified α-linolenic acid.
-
GC41900
13(S)-HpOTrE(γ)
13(S)-HpOTrE(γ) is a monohydroxy PUFA produced by the action of soybean lipoxygenase-1 (LO-1) on γ-linolenic acid.
-
GC62758
13-cis-Vitamin A palmitate
13-cis-Vitamin A palmitate (13-cis-Retinyl palmitate) is a 13-cis isomer formed by vitamin A palmitate in corn flakes.
-
GC41911
13-epi-12-oxo Phytodienoic Acid
13-epi-12-oxo Phytodienoic acid (13-epi-12-oxo PDA) is a lipoxygenase metabolite of α-linolenic acid in the leaves of green plants such as corn.
-
GC49759
13C17-Mycophenolic Acid
An internal standard for the quantification of mycophenolic acid
-
GC41206
14(S)-HDHA
Docosahexaenoic acid is a nutritionally-derived ω-3 fatty acid that is abundant in the brain and the retina and is thought to be important in early development and for therapeutic approaches to inflammatory disorders and cancer.
-
GC41100
14,15-dehydro Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-lipoxygenase pathway.
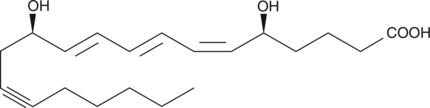
-
GC41145
14,15-Leukotriene C4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
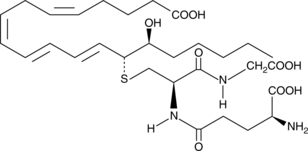
-
GC41148
14,15-Leukotriene D4
14,15-Leukotriene D4 (14,15-LTD4) is a member of an alternate class of LTs synthesized by a pathway involving the dual actions of 15- and 12-lipoxygenases (15- and 12-LOs) on arachidonic acid via 15-HpETE and 14,15-LTA4 intermediates.
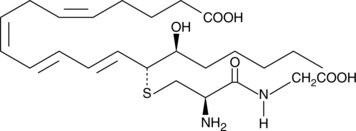
-
GC41150
14,15-Leukotriene E4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
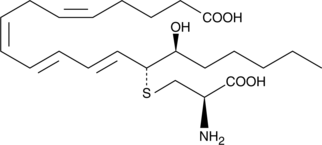
-
GC41415
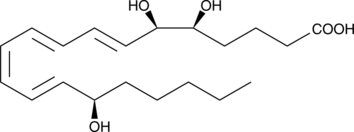
15(R)-Lipoxin A4
Lipid-derived lipoxins are produced at the site of vascular and mucosal inflammation where they down-regulate polymorphonuclear leukocyte recruitment and function.
-
GC40427
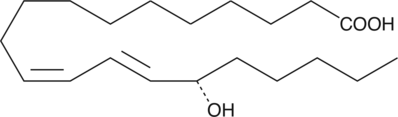
15(S)-HEDE
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO.
-
GC40373
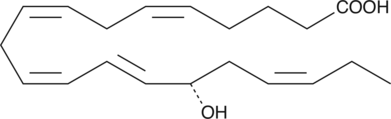
15(S)-HEPE
15(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 15-LO.
-
GC40451
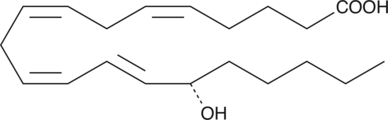
15(S)-HETE
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
-
GC41925
15(S)-HETE Ethanolamide
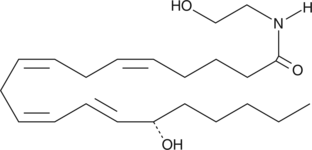
Arachidonoyl ethanolamide was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.
-
GC40839
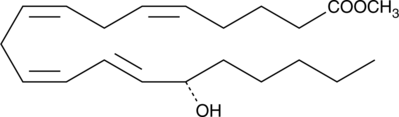
15(S)-HETE methyl ester
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE, a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
-
GC46442
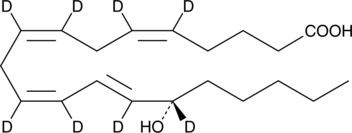
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
-
GC49894
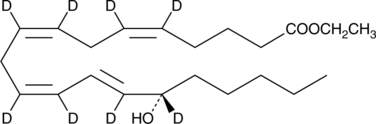
15(S)-HETE-d8 ethyl ester
An internal standard for the quantification of 15(S)-HETE ethyl ester
-
GC41927
15(S)-HETrE
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid.
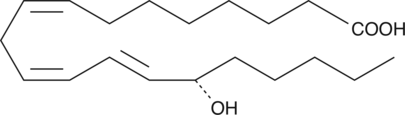
-
GC41403
15(S)-HpEDE
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid.
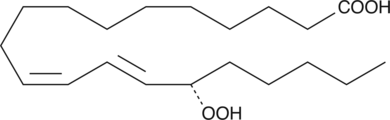
-
GC41101
15(S)-HpEPE
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid.
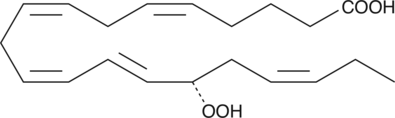
-
GC41124
15(S)-HpETE
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid.
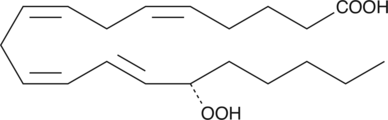
-
GC11988
15-acetoxy Scirpenol
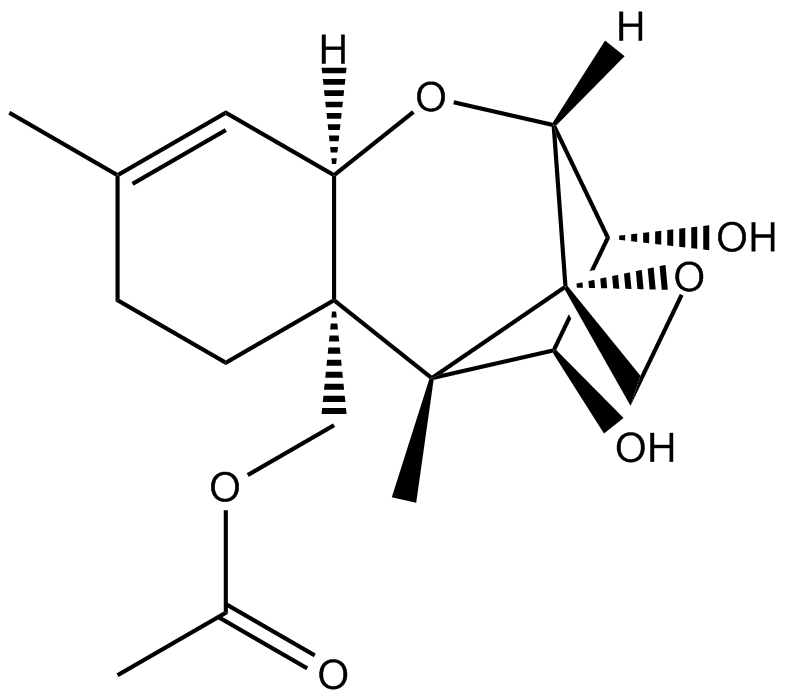
mycotoxin that induce apoptotic cell death
-
GC19442
15-Acetyldeoxy Nivalenol
15-Acetyldeoxy nivalenol is a trichothecene mycotoxin produced by certain species of the fungus Fusarium, particularly those found on cereal crops.

-
GC41937
15-keto-17-phenyl trinor Prostaglandin F2α ethyl amide
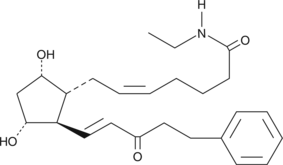
Bimatoprost is the Allergan trade name for 17-phenyl trinor prostaglandin F2α ethyl amide (17-phenyl trinor PGF2α ethyl amide), an F-series PG analog which has been approved for use as an ocular hypotensive drug.
-
GC41938
15-Lipoxygenase Inhibitor 1
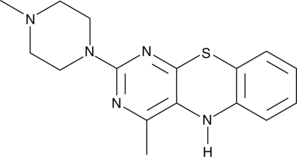
15-Lipoxygenase Inhibitor 1 is a selective inhibitor of 15-lipoxygenase, with an IC50 of 18 μM. 15-Lipoxygenase Inhibitor 1 has IC50s of 19.5 μM and 19.1 μM for soybean 15-lipoxygenase (SLO) and human 15-lipoxygenase-1 (15-LOX-1), respectively. 15-Lipoxygenase Inhibitor 1 has potential for the research of prostate cancer.
-
GC41940
15-OxoEDE
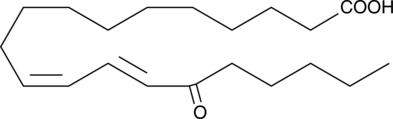
15-OxoEDE is produced by the oxidation of 15-HEDE.
-
GC40376
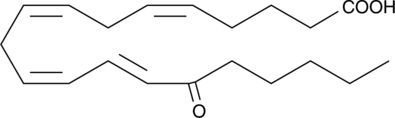
15-OxoETE
15-OxoETE is produced by oxidation of the 15-hydroxyl of 15-HETE.
-
GC41309
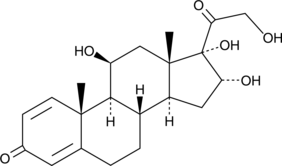
16α-hydroxy Prednisolone
16α-hydroxy Prednisolone is a stereoselective metabolite of the 22(R) epimer of the glucocorticoid budesonide.
-
GC35058
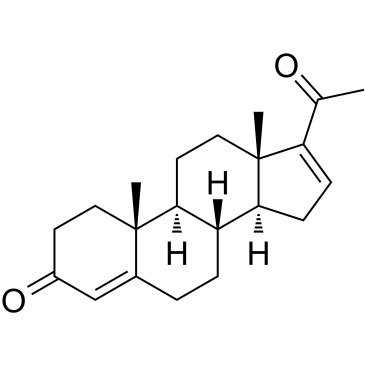
16-Dehydroprogesterone
16-Dehydroprogesterone is a steroidal progestin.
-
GC46451
16F16
A PDI inhibitor

-
GC45909
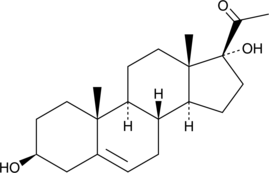
17α-hydroxy Pregnenolone
17α-hydroxy Pregnenolone is a pregnane steroid.
-
GC41300
17β-hydroxy Exemestane
17β-hydroxy Exemestane is the primary active metabolite of exemestane.

-
GC41951
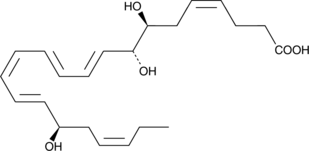
17(R)-Resolvin D1
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid and docosahexaenoic acid.
-
GC41227
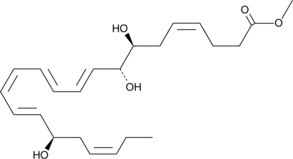
17(R)-Resolvin D1 methyl ester
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).
-
GC41952
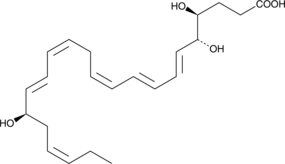
17(R)-Resolvin D4
17(R)-Resolvin D4 (17(R)-RvD4) is an aspirin-triggered epimer of RvD4 .
-
GC41208
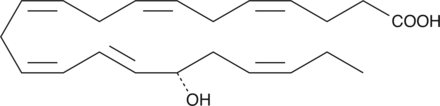
17(S)-HDHA
17(S)-HDHA is a primary mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, and mouse brain.
-
GC49356
17(S)-HDoTE
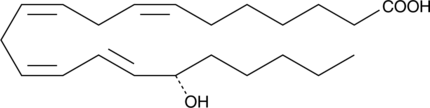
A metabolite of adrenic acid
-
GC40975
17(S)-HpDHA
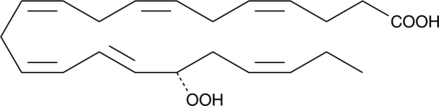
17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.
-
GC11720
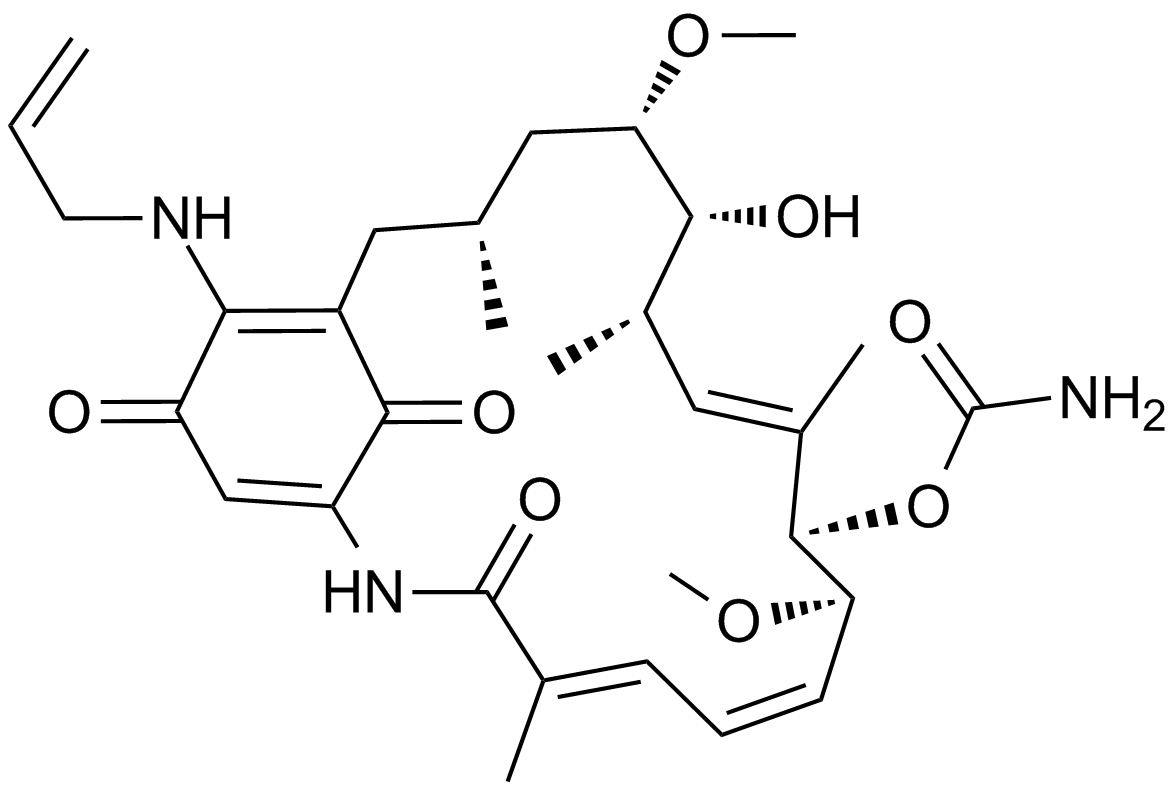
17-AAG (KOS953)
An inhibitor of Hsp90
-
GC17210
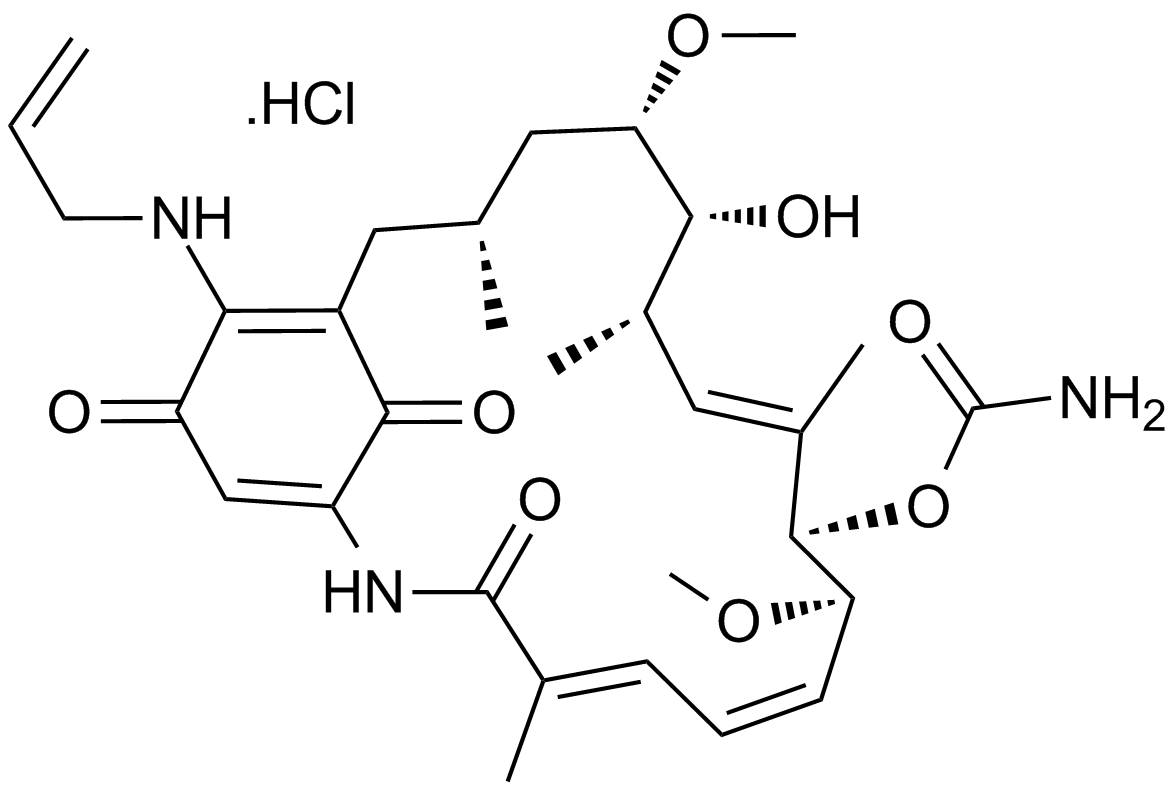
17-AAG Hydrochloride
Hsp90 inhibitor,geldanamycin analogue
-
GC41955
17-DMAG
17-DMAG (17-DMAG) is a potent inhibitor of Hsp90, binding to Hsp90 with an EC50 of 62 ± 29 nM.
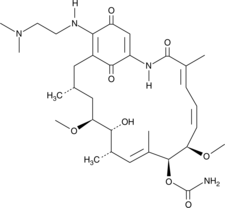
-
GC13044
17-DMAG (Alvespimycin) HCl
17-DMAG (Alvespimycin) HCl (17-DMAG hydrochloride; KOS-1022; BMS 826476) is a potent inhibitor of Hsp90, binding to Hsp90 with EC50 of 62±29 nM.

-
GC41529
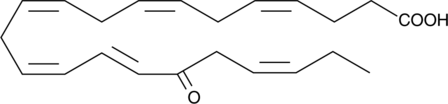
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic Acid
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic acid is a metabolite of lipoxygenase-mediated oxidation of DHA that is produced endogenously by aspirin-enhanced COX-2 activity.
-
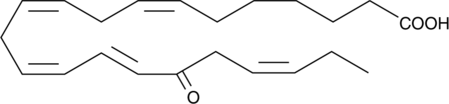
GC41209
17-oxo-7(Z),10(Z),13(Z),15(E),19(Z)-Docosapentaenoic Acid
Docosapentaenoic acid (DPA) is a ω-3 fatty acid found in fish oils.
-
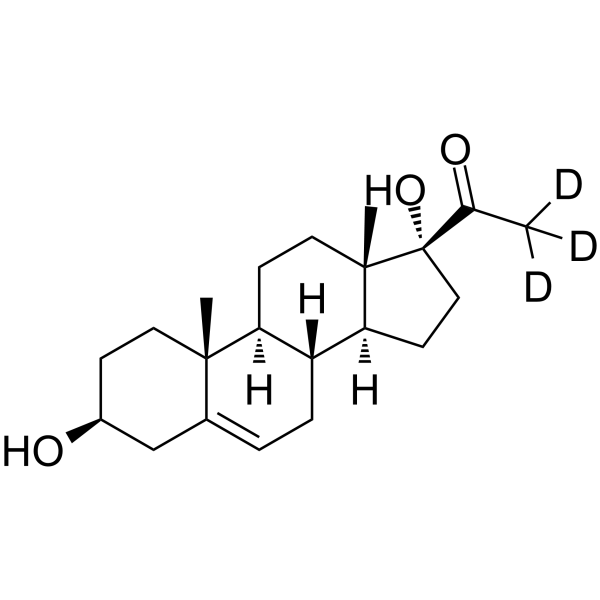
GC68426
17a-Hydroxypregnenolone-d3
-
GC35061
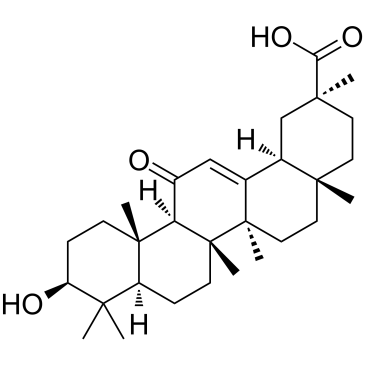
18α-Glycyrrhetinic acid
18α-Glycyrrhetinic acid, a diet-derived compound, is an inhibitor of NF-kB and an activator of proteasome, which serves as pro-longevity and anti-aggregation factor in a multicellular organism.
-
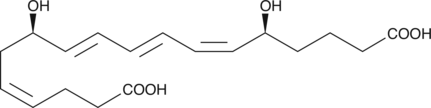
GC41980
18-carboxy dinor Leukotriene B4
18-carboxy dinor Leukotriene B4 (18-carboxy dinor LTB4) is a β-oxidation metabolite of LTB4.
-
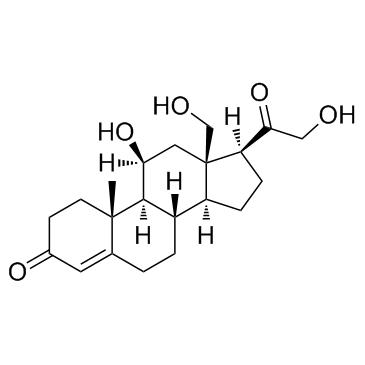
GC33818
18-Hydroxycorticosterone
18-Hydroxycorticosterone is a corticosteroid and a derivative of corticosterone, which can lead to serious electrolyte imbalances.
-
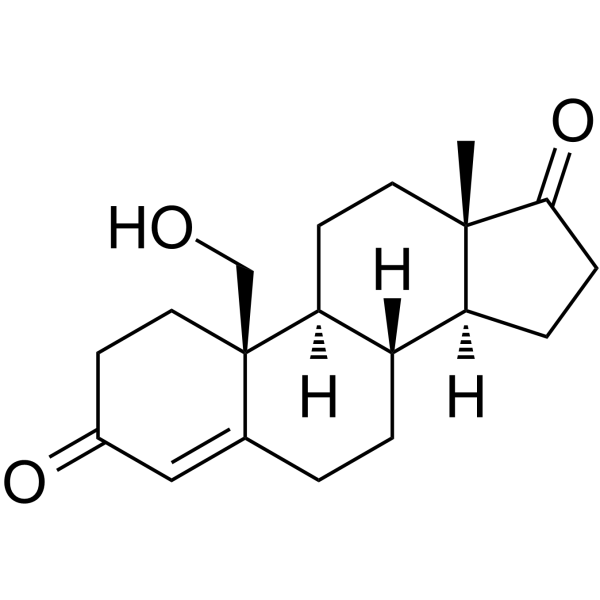
GC63603
19-Hydroxyandrost-4-ene-3,17-dione
-
GC39296
1G244
1G244 is a potent DPP8/9 inhibitor with IC50s of 12 nM and 84 nM, respectively. 1G244 does not inhibit DPPIV and DPPII. 1G244 induces apoptosis in multiple myeloma cells and has anti-myeloma effects.
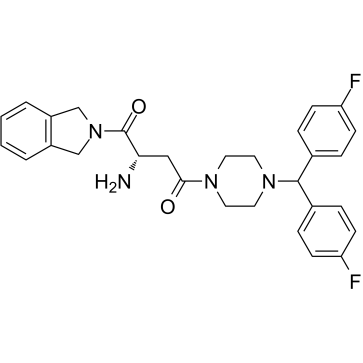
-
GC38359
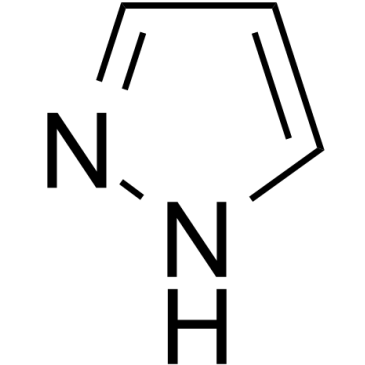
1H-pyrazole
1H-pyrazole is an endogenous metabolite.
-
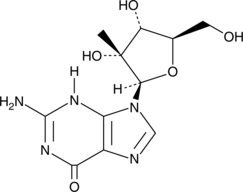
GC49823
2′-C-β-Methylguanosine
An active nucleoside metabolite of BMS-986094
-
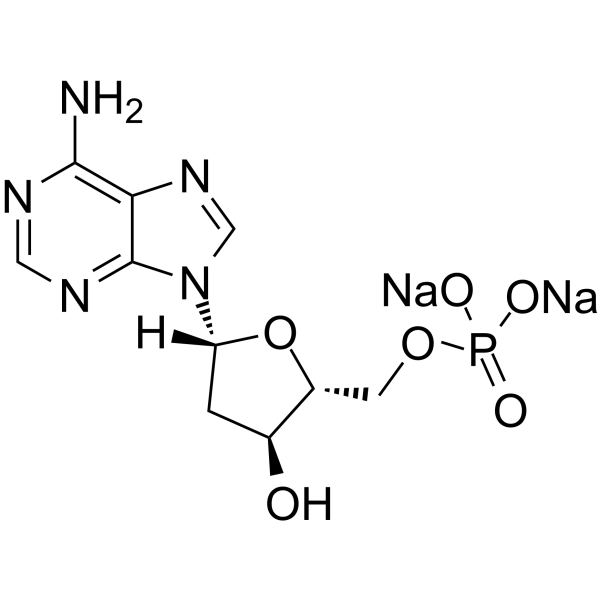
GC64738
2′-Deoxyadenosine 5′-monophosphate disodium
2′-Deoxyadenosine 5′-monophosphate disodium, a nucleic acid AMP derivative, is a deoxyribonucleotide found in DNA.
-
GC62772
2’-Deoxyadenosine-5’-triphosphate trisodium
2’-Deoxyadenosine-5’-triphosphate trisodium (dATP trisodium) is a nucleotide used in cells for DNA synthesis (or replication), as a substrate of DNA polymerase.
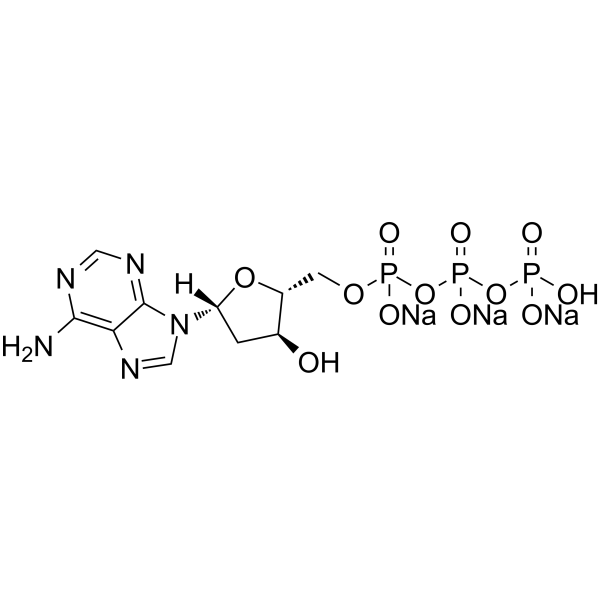
-
GC62774
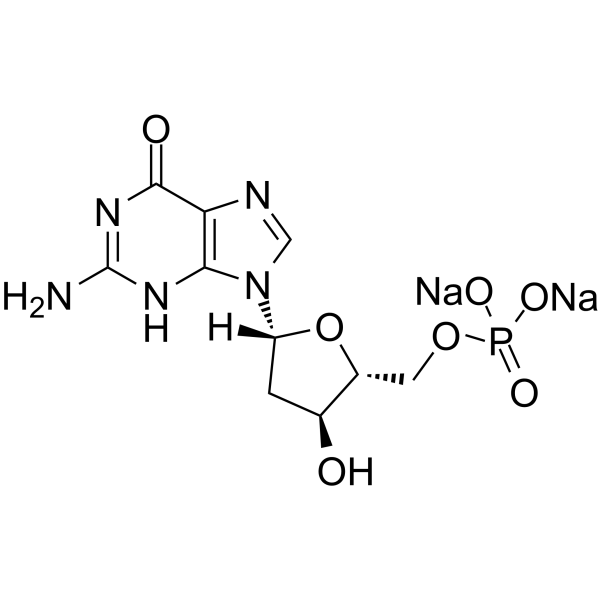
2’-Deoxyguanosine 5’-monophosphate disodium
2’-Deoxyguanosine 5’-monophosphate disodium (5′-dGMP disodium) is a mononucleotide having guanine as the nucleobase.
-
GC46508
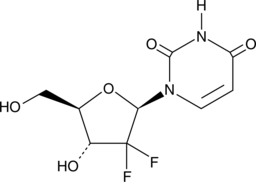
2',2'-Difluoro-2'-deoxyuridine
An active metabolite of gemcitabine
-
GC33622
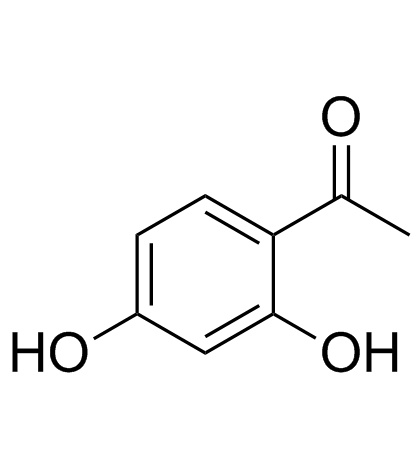
2',4'-Dihydroxyacetophenone
2',4'-Dihydroxyacetophenone (Resacetophenone) is acetophenone carrying hydroxy substituents at positions 2' and 4'.
-
GC60462
2',4'-Dimethylacetophenone
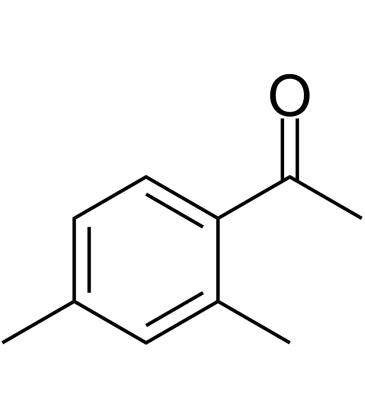
-
GC33605
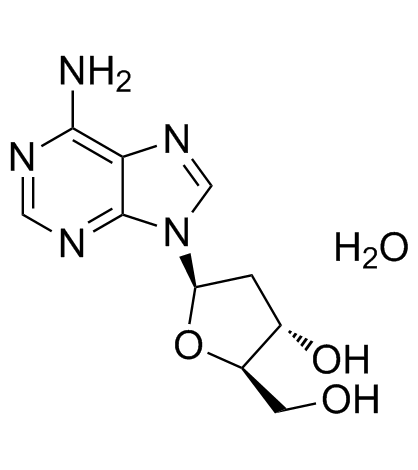
2'-Deoxyadenosine monohydrate
A deoxyribonucleoside
-
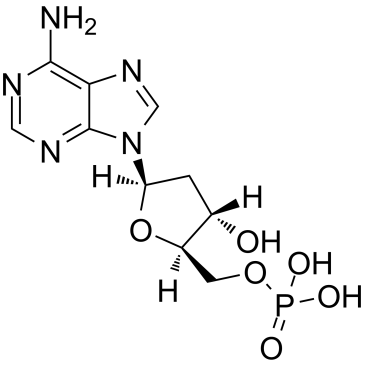
GC38258
2'-Deoxyadenosine-5'-monophosphate
A deoxynucleotide
-
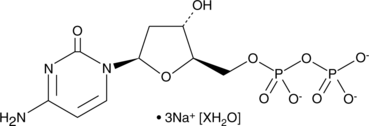
GC42150
2'-Deoxycytidine 5'-diphosphate (sodium salt hydrate)
2'-Deoxycytidine-5'-diphosphate (dCDP) trisodium is an endogenous metabolite.
-
GC17436
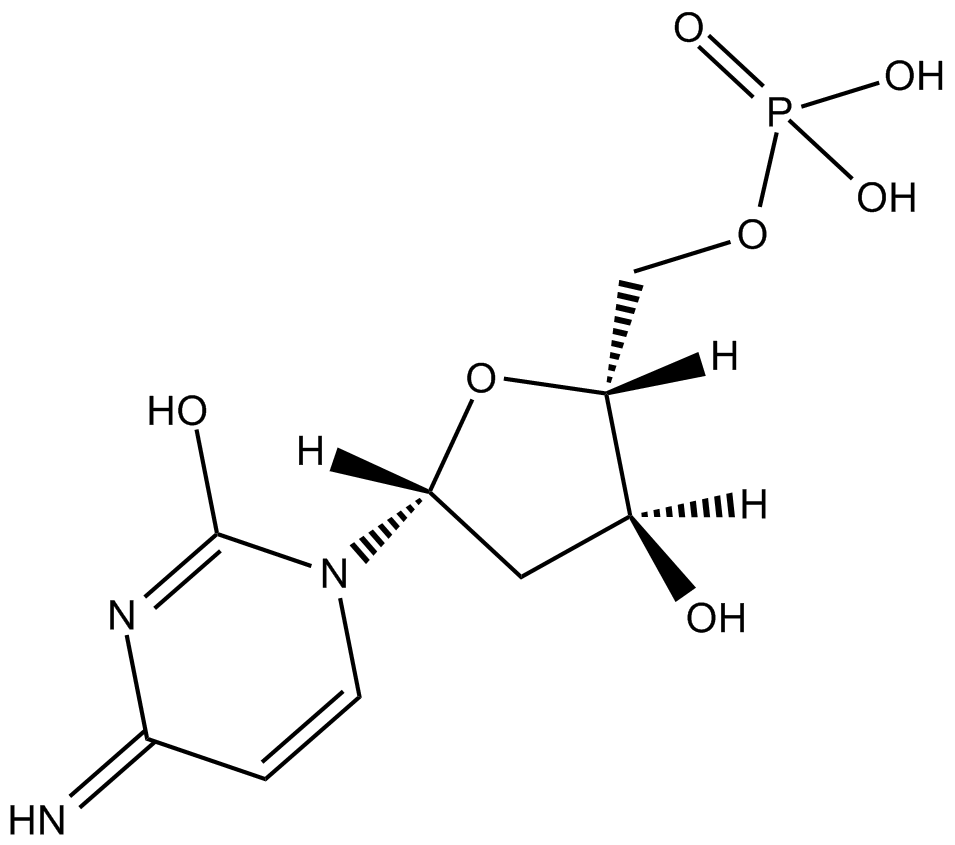
2'-Deoxycytidine-5'-monophosphoric acid
2'-Deoxycytidine-5'-monophosphoric acid is an endogenous metabolite.
-
GC48440
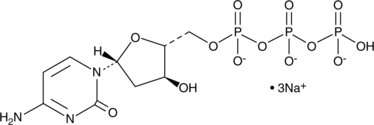
2'-Deoxycytidine-5'-triphosphate (sodium salt)
2'-Deoxycytidine-5'-triphosphate (sodium salt) (dCTP trisodium salt) is a nucleoside triphosphate that can be used for DNA synthesis.
-
GC10897
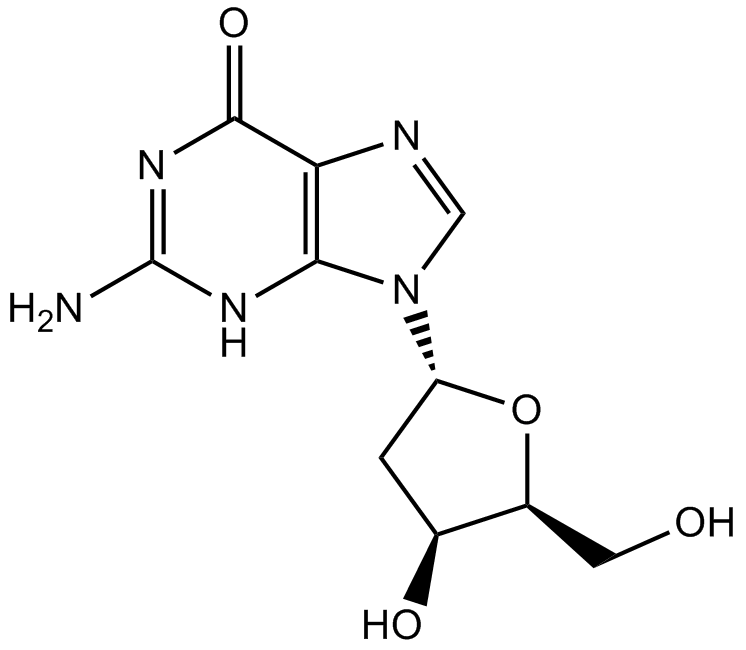
2'-Deoxyguanosine
2'-Deoxyguanosine
-
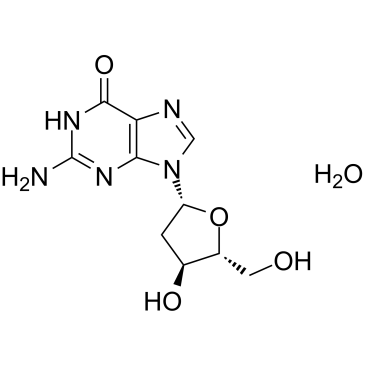
GC38191
2'-Deoxyguanosine monohydrate
A purine nucleoside with diverse biological activities