Resminostat hydrochloride (Synonyms: 4SC-201, RAS2410) |
| Catalog No.GC14243 |
An orally bioavailable HDAC inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
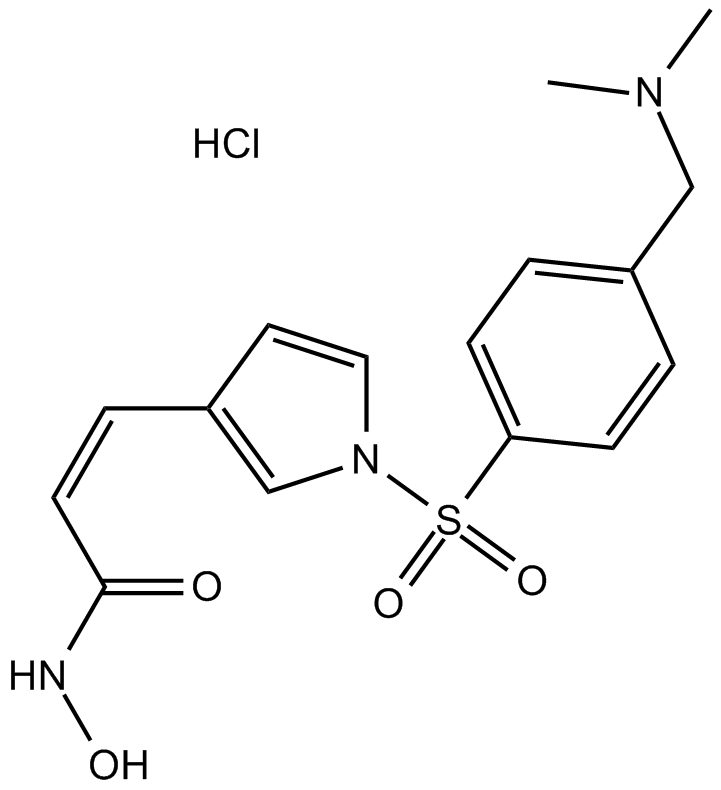
Cas No.: 1187075-34-8
Sample solution is provided at 25 µL, 10mM.
Resminostat is an inhibitor of histone deacetylase (HDAC) with IC50 values of 42.5nM, 50.1nM and 71.8nM, respectively against HDAC1, 3 and 6 [1].
As an inhibitor of HDACs, resminostat also inhibits HDAC8 with a weak activity (IC50=877nM). 5μM resminostat induces the acetylation of histone H4 in U266 myeloma cells. 10μM resminostat completely suppresses the cell growth in human myeloma cell lines, such as OPM-2, RPMI-8226 and U266. This inhibition is proved to be caused by the induction of apoptosis. In primary myeloma cells, resminostat also induces apoptosis of cells. Besides that, resminostat is proved to downregulate the expression of cell cycle proteins, including phosphorylated Rb, cdc25a, cyclin D1, Cdk4 and p53. In addition, resminostat is reported to be well tolerated, have no unexpected toxicities and do not cause clinically significant myelosuppression in the first-human study [1, 2].
References:
[1] Mandl-Weber S, Meinel FG, Jankowsky R, Oduncu F, Schmidmaier R, Baumann P. The novel inhibitor of histone deacetylase resminostat (RAS2410) inhibits proliferation and induces apoptosis in multiple myeloma (MM) cells. Br J Haematol. 2010 May;149(4):518-28.
[2] Brunetto AT, Ang JE, Lal R, Olmos D, Molife LR, Kristeleit R, Parker A, Casamayor I, Olaleye M, Mais A, Hauns B, Strobel V, Hentsch B, de Bono JS. First-in-human, pharmacokinetic and pharmacodynamic phase I study of Resminostat, an oral histone deacetylase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2013 Oct 1;19(19):5494-504.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















