Rifaximin (Xifaxan) (Synonyms: L 105SV, Rifamycin L 105) |
| Catalog No.GC16970 |
Rifaximin (Xifaxan), a gastrointestinal-selective antibiotic, binds the β-subunit of bacterial DNA-dependent RNA polymerase, resulting in inhibition of bacterial RNA synthesis.
Products are for research use only. Not for human use. We do not sell to patients.
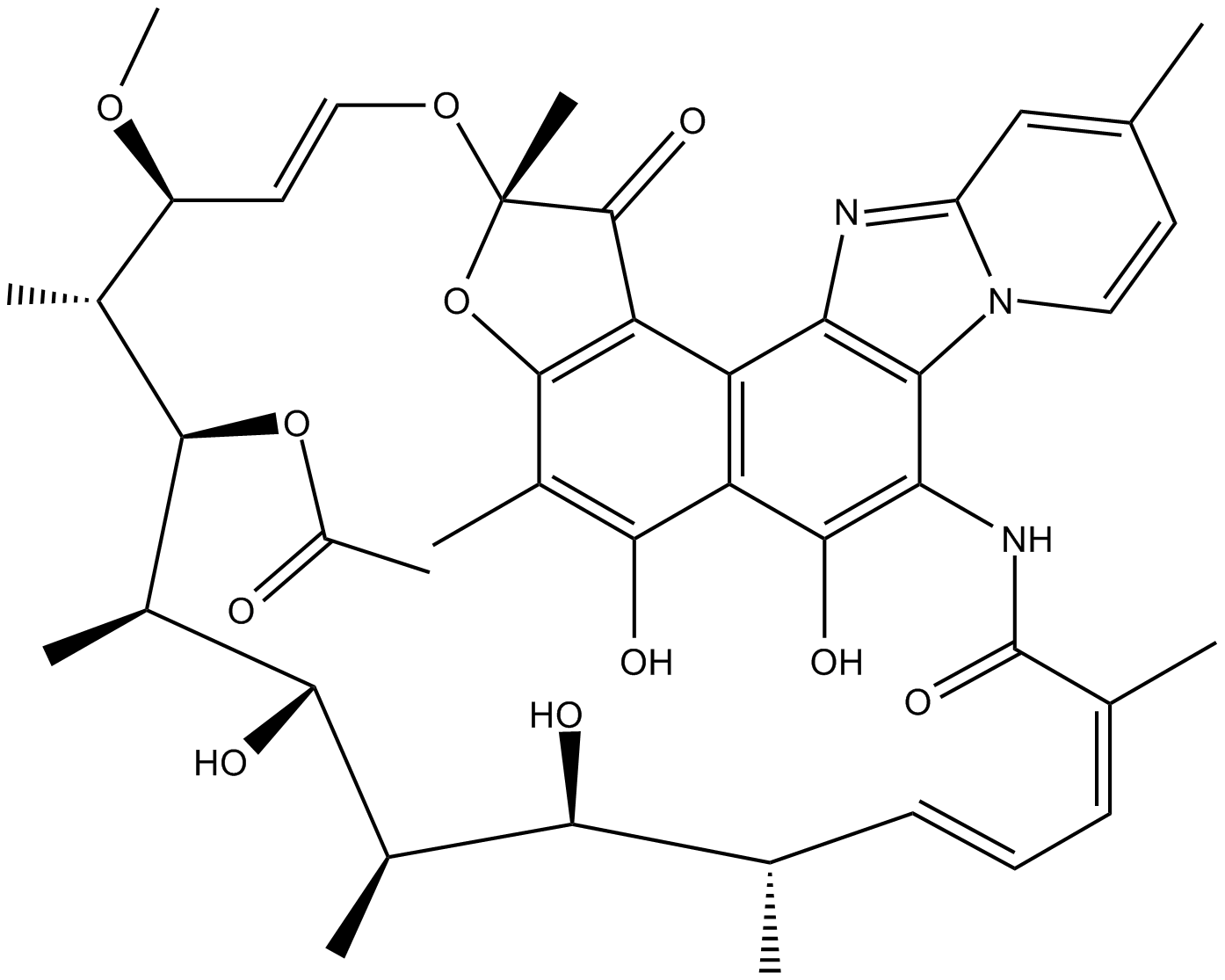
Cas No.: 80621-81-4
Sample solution is provided at 25 µL, 10mM.
Rifaximin, a rifamycin derivative, is a nonabsorbable antibiotic. Rifaximin inhibits RNA synthesis by binding the β subunit of the bacterial DNA-dependent RNA polymerase.
In vitro:Rifaximin, a gut-specific ligand for the human nuclear receptor pregnane-X receptor (PXR), contributes to the maintenance of the intestinal immune homeostasis. Rifaximin abrogates the binding of NF-κB caused by LPS. In human colon biopsies from inflammatory bowel diseases patients, exposure of rifaximin (100 μM) reduced mRNA levels of IL-8, Rantes, MIP-3α and TNFα induced by LPS stimulation [1]. Rifaximin acted on the β subunit of the deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase enzyme of bacteria to inhibit bacterial RNA synthesis. The susceptibility of Gram-positive organisms to rifaximin was greater than that of Gram-negative organisms [2]. In the DPX2 cell line transfected with stable recombinant human PXR expression, hPXR was significantly activated at RIFax concentrations over 1 μM and the EC50 was about 20 μM[3].
In vivo:In the small intestine of hPXR mice treated with rifaximin, several PXR target genes such as CYP3A11, GSTA1, MRP2, and OATP2 were up-regulated. Rifaximin treatment demonstrated no significant effect on hepatic PXR target genes in wild-type, Pxr-null, and hPXR mice [3]. In PXR-humanized mice, long-term administration of rifaximin for 6 months on the liver up-regulated the expression of hepatic genes related to triglyceride synthesis and lipid accumulation [4].
Clinical trials: Over a 6-month period, treatment with rifaximin maintained remission from hepatic encephalopathy effectively. Rifaximin treatment also significantly reduced the risk of hospitalization involving hepatic encephalopathy [5]. Among patients with irritable bowel syndrome (IBS) without constipation, treatment with rifaximin for 2 weeks provided significant relief of IBS symptoms, bloating, abdominal pain, and loose or watery stools [6]. Rifaximin could improve the symptom in IBS patients, such as abdominal bloating and flatulence [7].
References:
[1]. Mencarelli A, Renga B, Palladino G, et al. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells[J]. European journal of pharmacology, 2011, 668(1): 317-324.
[2]. Gillis J C, Brogden R N. Rifaximin[J]. Drugs, 1995, 49(3): 467-484.
[3]. Ma X, Shah Y M, Guo G L, et al. Rifaximin is a gut-specific human pregnane X receptor activator[J]. Journal of Pharmacology and Experimental Therapeutics, 2007, 322(1): 391-398.
[4]. Cheng J, Krausz K, Tanaka N, et al. Chronic exposure to rifaximin causes hepatic steatosis in pregnane X receptor-humanized mice[J]. Toxicological Sciences, 2012: kfs211.
[5]. Bass N M, Mullen K D, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy[J]. New England Journal of Medicine, 2010, 362(12): 1071-1081.
[6]. Pimentel M, Lembo A, Chey W D, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation[J]. New england journal of medicine, 2011, 364(1): 22-32.
[7]. Sharara A I, Aoun E, Abdul-Baki H, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence[J]. The American journal of gastroenterology, 2006, 101(2): 326-333.
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















