S-Ruxolitinib (INCB018424) |
| Catalog No.GC17020 |
Products are for research use only. Not for human use. We do not sell to patients.
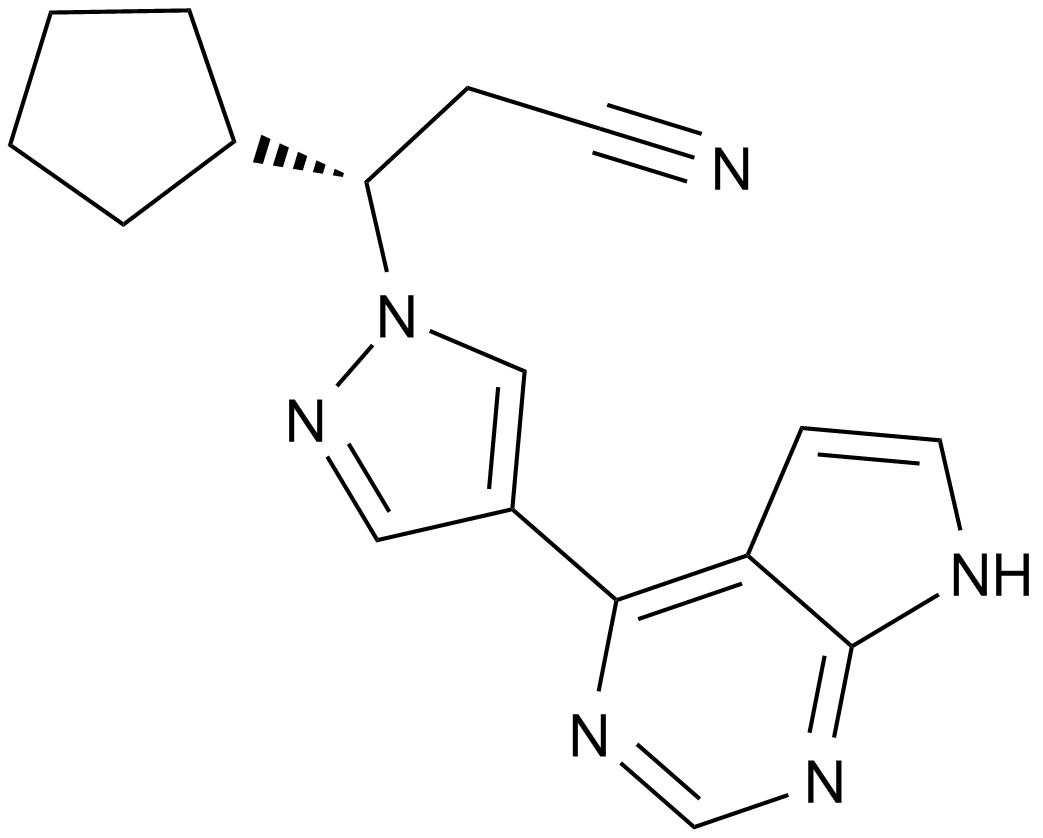
Cas No.: 941685-37-6
Sample solution is provided at 25 µL, 10mM.
S-Ruxolitinib is the chirality of INCB018424, is a potent and selective small-molecule Janus kinase 1 (JAK1) and JAK2 inhibitor. It was initially developed to target the constitutive activation of the JAK-STAT pathway. Janus kinases (JAKs) are a family of cytoplasmic tyrosine kinases that mediates signals from the receptors for various cytokines and growth factors that have a key role in haematopoiesis and immune function. Ruxolitinib maintains its anti-JAK activity by competitive inhibition of the ATP-binding catalytic site of the kinase domain. Ruxolitinib is well absorbed at >95%. Exposure of JAK2V617F-positive Ba/F3 cells to ruxolitinib iss shown to result in reduced cellular proliferation.
Reference
[1].Ruben A. Mesa, Uma Yasothan, Peter Kirkpatrick. Ruxolitinib. Nature Reviews Drug Discovery. 2012; 11: 103-104
[2].John Mascarenhas, Ronald Hoffman. Ruxolitinib: The First FDA Approved Therapy for the Treatment of Myelofibrosis. Clinical Cancer Research. 2012; 18(11): 3008 - 3014
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















