ML385
It is defined as a novel synthetic compound that can block transcription activity by inhibiting the action of Nrf2. Nrf2 is also called nuclear factor erythroid 2-related factor 2. The nrf2 signaling pathway gives the therapeutic approach to the researchers to treat the various kinds of inflammatory, metabolic, cardiovascular, and neuro-inflammatory disorders. It is because it initiates the homeostatic response against the changes in the metabolism and inflammatory responses.
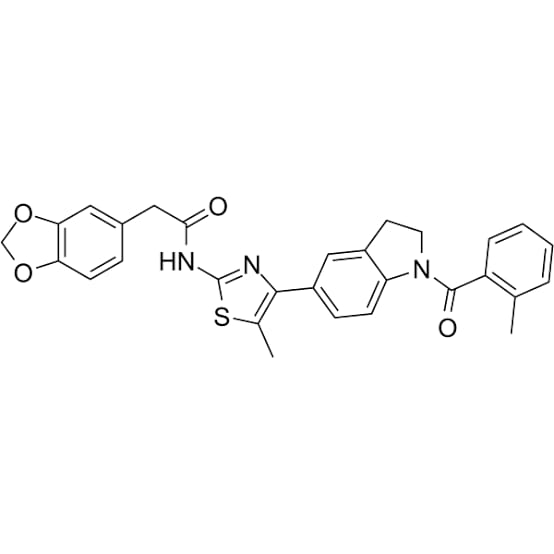
Figure 1: Structure of ML385
Apart from this study, the Nrf2 activation signaling pathway plays an important role in the survivability and progression of tumors. Thus, a molecule that can block the transcription activity of NRF2 can be used as an anti-tumor drug for the treatment of various kinds of cancers. These NRF2 factors are derived from the NFE2L2 gene. It belongs to the cap “n” family of transcription factors. In general, its domains range from Neh1-7. The domains at the C terminal play an important role in the formation of the heterodimer by interacting with other proteins. Similarly, these heterodimers then regulate the homeostatic response for the regulation of inflammation and redox metabolism. Different studies explained that ML385 not only inhibits the transcriptional activity of the NRF2 thus preventing the survivability and progression of the tumors but also can sensitize the cells which are deficient in KEAP1 to various chemotherapeutic drugs, radiotherapy, and carboplatin. As we know that NRF2 has DNA binding domains in its structure. ML385 binds to the DNA binding domain of the NRF2, which in return prevents the binding of NRF2 with ARE’s enhancers. This ARE enhancer is similar to other enhancers includes API. so there is the need for other advanced studies to determine, whether ML385 has a selective role for NRF2 or it can inhibit other transcription factors like bZip which play part in chemoresistance.
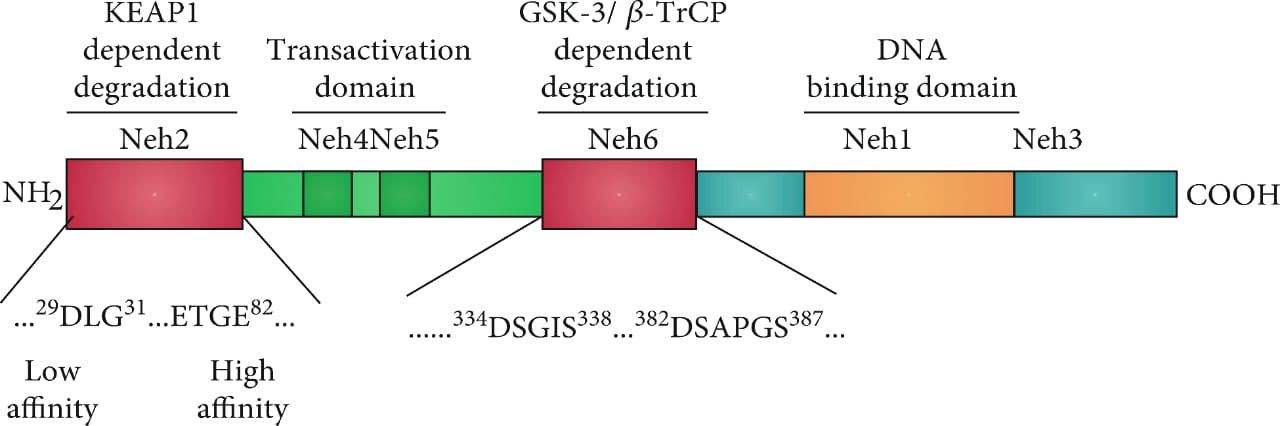
Figure 2: structural representation of NRF2 Domains.
As we discussed the activation of NRF2 is involved in the survivability and progression of the tumor. Studies stated that kletch like ECH-associated protein I, negatively regulate the activation of NRF2 by directly binding to it, which in return leads to proteasomal degradation. They also explained that this KEAP1 loss of function mutation is associated with non-small cell lung cancer (NSCLC) by consecutively activating the NRF2. This is seen particularly in adenocarcinomas. Similarly, the activating mutations which occur in the NRF2 are also associated with squamous cell lung carcinoma, because this leads to the improper interaction between NRF2 and KEAP1. This improper interaction between NRF2 and KEAP1 is because of mutation not only found in various types of lung cancer, but also different prostate, esophageal, and renal cancers. Hence this activation of the NRF2 in NSCLC directly provides protective effects to tumor cells as a resistance against chemotherapy and radiotherapy. Along with this study, different in-vivo and in-vitro studies stated that inhibition of NRF2 mediated through RNAi can increase the sensitization of the tumor cells for chemo and radiotherapy.
Furthermore, they treated the A549 cells with ML385, to check whether the inhibition of the transcriptional activity of NRF2 is caused by ML385 or not. After the experiment, they found that the inhibition of the transcriptional activity of NRF2 and associated genes by ML385 is time and dose-dependent. They found that a minimum 5μM concentration of ML385 was sufficient enough to inhibit the NRF2 activation and their target genes. They also found that the maximum decrease in the transcriptional activity was measured at 72hr along with the reduction of mRNA NRF2 level. Apart from this, they again treated the A549 cells at 48 and 72hr intervals with ML385. Again, they noticed the time and dose-dependent reduction of the genes for glucose metabolism, recycling enzymes, synthesis of glutathione, NQO1 activity of the enzyme, GSH, and cellular antioxidant capacity of the cell. The same results were observed for the H460 cell lines of carcinoma having KEAP1 mutation. At last, they treat the EBC1 cell lines of squamous cell lung carcinoma having the gain of function mutation in NRF2 with ML385. In this experiment they found that there is a Dose-dependent reduction of the NRF2 activation and gene expression related to glutathione genes. After that, they again treated the A549 & H460 cell lines with ML385 in combination with the chemotherapeutic drugs for 72 hours. They found a reduction in the number of colonies as compared to the single use of ML385. Luminescence-based assay for colonies revealed that, it is because of the increase in the apoptosis of the cancer cells mediated through caspase3 enzymes. All these results explained that the ML385 is selective in its action as cytotoxic for nonsmall cell lung cancers along with the increase in the toxicity effects of chemotherapeutic drugs, particularly for carboplatin and paclitaxel.
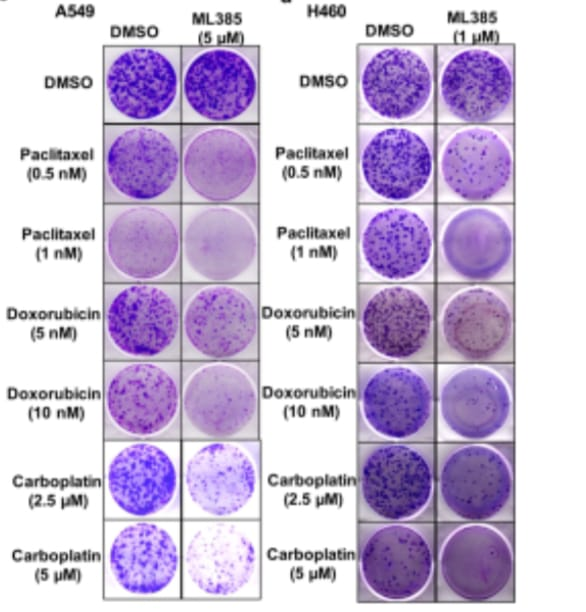
Figure 3: luminescence-based analysis of NSCLC with KEAP1 mutation treated with ML385 for determining the cytotoxicity of chemotherapeutic drugs.
As we discussed earlier that NRF2 helps the cancer cells for their proliferation and survivability. It is done by various mechanisms. First of all, Nrf2 regulates the reactive oxygen species in the cancer cells because of the ability to redox homeostasis. As cancer cells have a high anabolic rate and energy demand as compared to normal cells. So NRF2 along with the ATF4 helps the cancer cells to fulfill their anabolic demands by activating the biosynthetic pathway of glycine/serine. Similarly, LUSC has activated the PI3K-AKT pathway because of genetic alteration. So NRF2 can direct the glucose in anabolism through the PI3K-AKT pathway, because this pathway can increase the NRF2 protein level. Along with these mechanisms, NRF2 can upregulate the PI3K-mTOR pathway, through which, it can increase the survivability and progression of cancer cells, particularly in lung squamous cell carcinoma. So ML385 can be used as a therapeutic drug to inhibit the activation of NRF2 to inhibit the growth of cancer cells. The study found that ML385 can inhibit the XDO377 LUSC organoids by inhibiting the transcriptional activity of NRF2 and NQO1 activity. In LUSC, the PI3K pathway is upregulated because of genetic alterations like point mutation. Similarly, NRF2 activation also promotes the PI3K pathway. A study conducted on the combined use of BKM120 and ML385 in LUSC revealed that the combination of these two inhibitors BMK120 for PI3K inhibition and ML385 for NRF2 inhibition can suppress the growth of squamous lung cancer cells by inhibiting the 4EBP1 and AKT phosphorylation.ML385 can also cause the downregulation of RagD, which in return reduces the level of recruitment of mTORC1, thus reducing the activation of lysosomes.













Comentarios