Lenvatinib (E7080) (Synonyms: E-7080, ER-203492-00) |
| Catalog No.GC15454 |
Lenvatinib (E7080) (E7080) es un inhibidor oral de la tirosina quinasa multidirigido que inhibe VEGFR1-3, FGFR1-4, PDGFR, KIT y RET, muestra potentes actividades antitumorales.
Products are for research use only. Not for human use. We do not sell to patients.
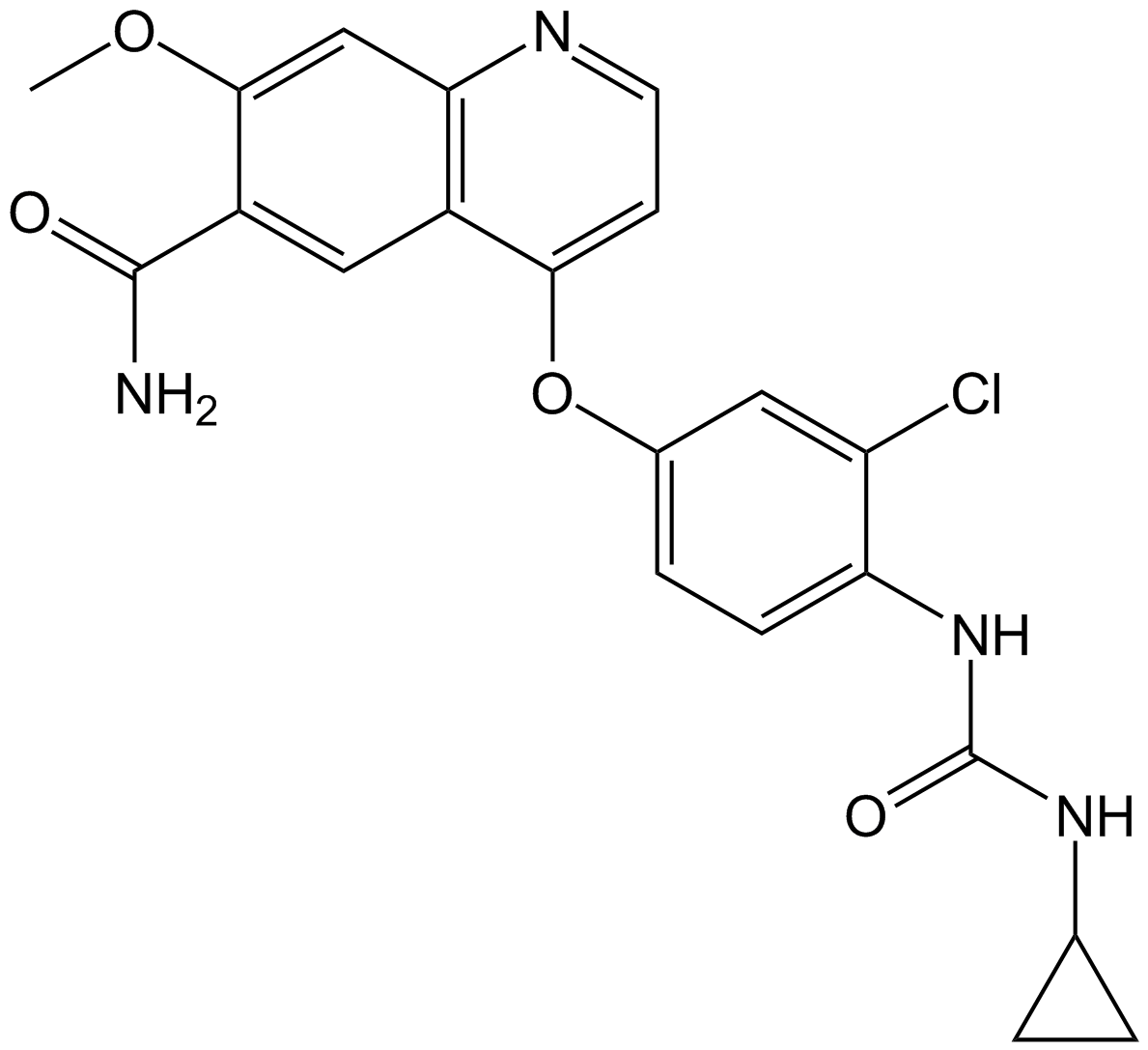
Cas No.: 417716-92-8
Sample solution is provided at 25 µL, 10mM.
E7080, known as lenvatinib, is an oral multitargeted tyrosine kinase inhibitor including VEGF, FGF and SCF receptors that has been shown to improve the survival rate of patients with radioiodine-refractory thyroid cancer. Lenvatinib (E7080) had antitumor activity against HCC PDX models, likely through its potent anti-angiogenic activity [1].
Lenvatinib (E7080) inhibited Flt-1, KDR, Flt-4 with IC50 values of 22, 4.0 and 5.2 nM, respectively. Lenvatinib (E7080) inhibited FGFR1 and FDGFR tyrosine kinases. In addition to these kinases, Lenvatinib (E7080) also inhibited KIT kinase with an IC50 value of 100 nM [2]. The half-maximal inhibitory concentrations (IC50 ) for Lenvatinib (E7080) treatment of 8505C and TCO1 cells were 24.26 and 26.32 μM, respectively [3].
The novel multi-targeted kinase inhibitor Lenvatinib (E7080), which inhibited both KDR and KIT kinases, showed a more potent antitumor efficacy against H146 tumor than imatinib. Oral administration of Lenvatinib (E7080) inhibited the growth of H146 tumor at 30 and 100 mg/kg in a dose-dependent manner and caused tumor regression at 100 mg/kg [2]. Lenvatinib (E7080) at 10 and 30 mg/kg inhibited the tumor growth of both PDXs, LI0050 and LI0334 [1]. Lenvatinib (E7080), as compared with placebo, was associated with significant prolongation of progression-free survival and an improved response rate (64.8% vs. 1.5%) among patients with iodine-131–refractory differentiated thyroid cancer [4].
References:
[1].Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018 Jun;7(6):2641-2653.
[2].Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008 Feb 1;122(3):664-71.
[3].Enomoto K, Hirayama S, Kumashiro N, Jing X, Kimura T, Tamagawa S, Matsuzaki I, Murata SI, Hotomi M. Synergistic Effects of Lenvatinib (E7080) and MEK Inhibitors against Anaplastic Thyroid Cancer in Preclinical Models. Cancers (Basel). 2021 Feb 18;13(4):862.
[4].Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015 Feb 12;372(7):621-30.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




