Sulindac sulfide (Synonyms: cis-Sulindac sulfide) |
| Catalog No.GC15059 |
COX inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
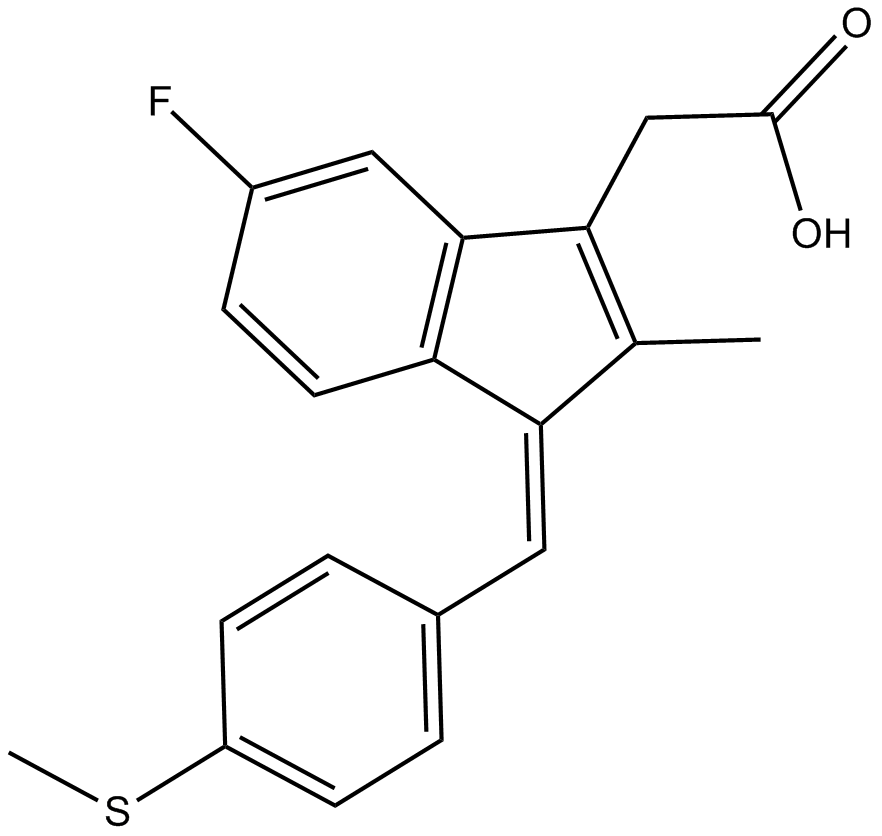
Cas No.: 49627-27-2
Sample solution is provided at 25 µL, 10mM.
Sulindac is a non-steroidal anti-inflammatory drug that has an extensive epidemiology documenting reduced human colorectal cancer. In mouse models, sulindac was found not only to inhibit the enzymatic activity of polyp-associated COX-2, but also to downregulate the expression of colonic COX-2 protein to control levels.[1] Sulindac sulfide is a metabolite of sulindac that has diverse activities.[2],[3] It inhibits both COX-1 and COX-2 (IC50s = 1.9 and 1.21 µM, respectively), whereas the parent compound, sulindac, is much less effective (IC50 = 58 µM for COX-2 and > 100 µM for COX-1).[4] Sulindac sulfide also inhibits aldose reductase (IC50 = 279 nM), blocking NADPH-dependent reduction of glucose to sorbitol, and reducing type 2 diabetic complications.[5] It increases the expression and activity of NAD(P)H quinone oxidoreductase [1].[6] Sulindac sulfide inhibits colorectal cancer growth both in vitro and in vivo.[7]
Reference:
[1]. Boolbol, S.K., Dannenberg, A.J., Chadburn, A., et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Research 56, 2556-2560 (1996).
[2]. Kitamura, S., and Tatsumi, K. In vitro metabolism of sulindac and sulindac sulfide: Enzymatic formation of sulfoxide and sulfone. Japanese Journal of Pharmacology 32(5), 833-838 (1982).
[3]. Brunell, D., Sagher, D., Kesaraju, S., et al. Studies on the metabolism and biological activity of the epimers of sulindac. Drug Metabolism and Disposition 39(6), 1014-1021 (2011).
[4]. Warner, T.D., Giuliano, F., Vojnovic, I., et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Nat. Acad. Sci. USA 96(13), 7563-7568 (1999).
[5]. Zheng, X., Zhang, L., Zhai, J., et al. The molecular basis for inhibition of sulindac and its metabolites towards human aldose reductase. FEBS Letters 586(1), 55-59 (2012).
[6]. Kung, H.N., Weng, T.Y., Liu, Y.L., et al. Sulindac compounds facilitate the cytotoxicity of β-lapachone by up-regulation of NAD(P)H quinone oxidoreductase in human lung cancer cells. PLoS One 9(2), 1-15 (2014).
[7]. Williams, C.S., Goldman, A.P., Sheng, H., et al. Sulindac sulfide, but not sulindac sulfone, inhibits colorectal cancer growth. Neoplasia 1(2), 170-176 (1999).
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















