Sunitinib malate (Synonyms: SU 11248,SU11248,SU-11248,Sunitinib) |
| Catalog No.GC14683 |
A multi-kinase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
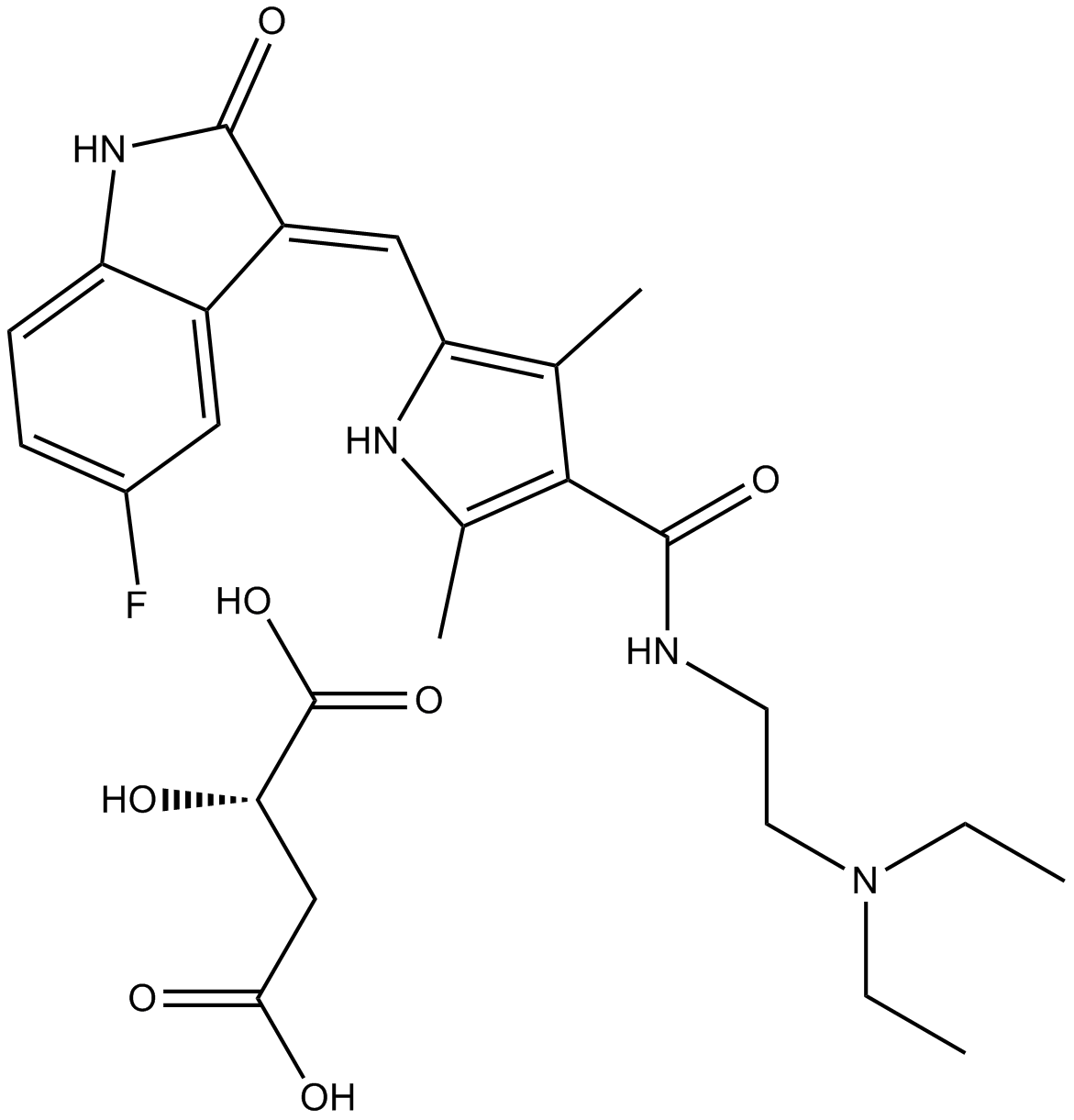
Cas No.: 341031-54-7
Sample solution is provided at 25 µL, 10mM.
Sunitinib malate, also called sunitinib, is a novel, oral, multi-targeted , small molecule oxindole tyrosine kinase inhibitor which inhibits multiple receptor tyrosine kinases including platelet-derived growth factor receptor ( and (, vascular endothelial growth factor receptor 1, 2 and 3, c-KIT, FLT3 kinase, colony-stimulating factor 1 receptor and RET kinase [2][3] [4]. The IC50 of sunitinib is approximately 10-20 ng/ml to NB cell lines, which is within the clinically relevant human trough serum concentration (50-100 ng/ml) [1].
Receptor tyrosine kinases activated a number of different intracellular signaling pathways [5].
In neuroblastoma (NB) cell lines, SKN-BE (2), NUB-7, SH-SY5Y and LAN-5, sunitinib significantly inhibited cell proliferation after a treatment for 48 hours, in a concentration-dependent manner [1].
Treatment with 20, 30 or 40 mg/kg of sunitinib made NOD/SCID mice inoculated with xenograft tumor cells show significant reduction (P <0.05) in primary tumor growth (%T/C: 49% for SK-N-BE (2) and 55% for NB12 tumor, T/C: average treated tumor mass/average control tumor mass). Treatment with different doses of sunitinib (20, 30 or 40 mg/kg) for 14 days resulted in a dramatic decrease in the numbers and size of metastatic sites and a significant difference in liver weight in mice injected intravaneously with 106 SK-N-BE(2) cells for 7 days compared with the control group [1].
References:
[1]. Libo Zhang, Kristen M. Smith, Amy Lee Chong, et al. In Vivo Antitumor and Antimetastatic Activity of Sunitinib in Preclinical Neuroblastoma Mouse Model. Neoplasia, 2009, 11: 426-435.
[2]. Hassane Izzedine, Irina Buhaescu, Olivier Rixe, et al. Sunitinib malate. Cancer Chemother Pharmacol, 2007, 60: 357-364.
[3]. M. L. Telli, R. M. Witteles, G. A. Fisher, et al. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Annals of Oncology, 2008, 19: 1613–1618.
[4]. Edwin P. Rock, Vicki Goodman, Janet X. Jiang, et al. Food and Drug Administration Drug Approval Summary: Sunitinib Malate for the Treatment of Gastrointestinal Stromal Tumor and Advanced Renal Cell Carcinoma. The Oncologist, 2007, 12: 107-113.
[5]. C. J. Marshall. Specificity of Receptor Tyrosine Kinase Signaling: Transient versus Sustained Extracellular Signal-Regulated Kinase Activation. Cel, 1995, 80: 179-185.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




