Suramin hexasodium salt (Synonyms: BAY 205, Germanin, NF 060) |
| Catalog No.GC16832 |
Suramin hexasodium salt(Suramin hexasodium salt) is a reversible and competitive protein-tyrosine phosphatases (PTPases) inhibitor.
Products are for research use only. Not for human use. We do not sell to patients.
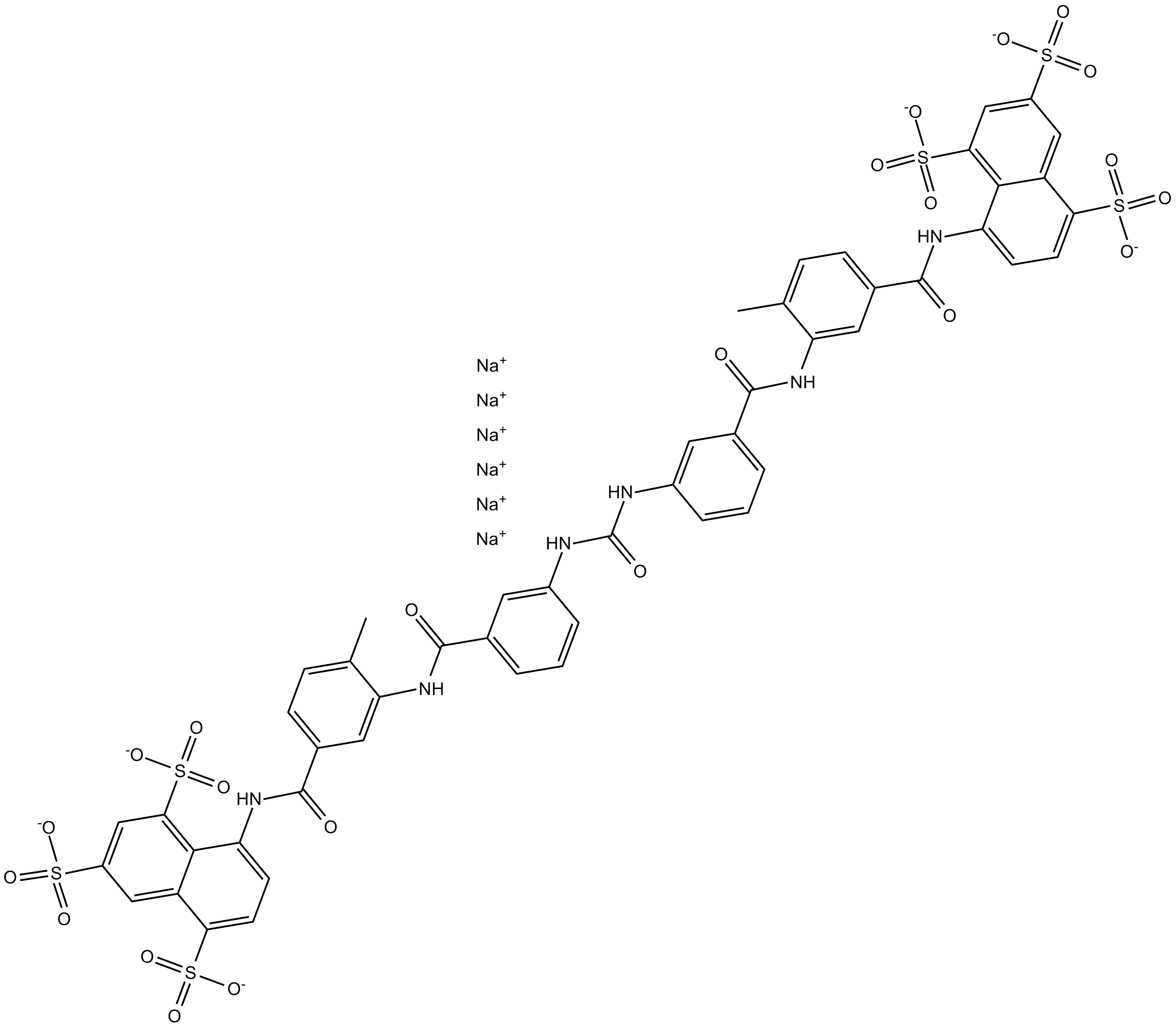
Cas No.: 129-46-4
Sample solution is provided at 25 µL, 10mM.
Suramin hexasodium salt is a polysulfonated naphthylurea with various biological activities. Suramin hexasodium salt is a DNA topoisomerase II inhibitor with an IC50 of 5 μM [1].
Suramin hexasodium salt at 300–600 μg/ml significantly inhibited HO-8910 PM and HeLa cell growth at 24 h, in both a time-dependent and dose-dependent manner, with an IC50 of 320 μg/ml and 475 μg/ml, respectively. Suramin hexasodium salt at 300 μg/ml significantly decreased the expression of Hpa mRNA (P < 0.005) and protein (P < 0.005) in both HO-8910 PM and HeLa cells at 48 h [2]. Suramin hexasodium salt shows antiviral activity against the newly emerged virus strain SARS-CoV-2 [3,4].Solution based assays of RdRp inhibition determined that the half-maximal inhibition concentration (IC50) of suramin hexasodium salt is 0.26 µM, Cell-based experiments indicated that suramin hexasodium salt was able to inhibit SARS-CoV-2 duplication in Vero E6 cells with a half-maximal effective concentration (EC50) of roughly 2.9 µM [3].Suramin hexasodium salt treatment of infected Vero E6 cells led to a reduction in extracellular viral RNA levels of up to 3 log. The highest concentration of compound that was used proved harmless to the cells; also, cytotoxicity was observed previously only above 5 mM. Suramin hexasodium salt also displayed antiviral efficacy in a human lung epithelial cell line, and we observed a >2 log reduction in levels of infectious virus progeny in suramin hexasodium salt -treated cells (CC50/EC90 = >55) [4].
Suramin hexasodium salt has therapeutic effects on CHIKV-infected mice. Suramin hexasodium salt treatment ameliorated foot swelling and reduced inflammatory infiltration, which corresponded to reduced viremia and viral antigen expression in infected tissues. Suramin hexasodium salt induces a dose-dependent reduction in foot swelling in CHIKV 0810bTw-infected mice, and the vary degrees of decreased viremia that was detected in suramin hexasodium salt -treated mice further confirmed therapeutic effects of this drug. In the time-related assay, a single dose of 2 mg suramin hexasodium salt (at 4 h pre-infection) or double doses of 2 mg suramin hexasodium salt (at 1 dpi and 3 dpi) significantly decreased disease score and viremia compared to mock-treated mice.
References:
[1]. Bojanowski K, et al. Suramin is an inhibitor of DNA topoisomerase II in vitro and in Chinese hamster fibrosarcomacells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3025-9.
[2]. Li, H.; Li, H.; Qu, H.; et al. Suramin inhibits cell proliferation in ovarian and cervical cancer by downregulating heparanase expression. Cancer Cell Int. 2015, 15, 1–11
[3]. Yin, W. et al. Structural basis for inhibition of the SARS-CoV-2 RNA polymerase by suramin. Nat. Struct. Mol. Biol. 28, 319–325 (2021).
[4]. Salgado-Benvindo, C., Thaler, M., Tas, A., Ogando, N. S., et al. Suramin inhibits SARS-CoV-2 infection in cell culture by interfering with early steps of the replication cycle. 2020. Antimicrob. Agents Chemother. 20, DOI: 10.1128/AAC.00900-20
[5]. Kuo SC, Wang YM, Ho YJ, Chang TY, Lai ZZ, Tsui PY, Wu TY, Lin CC. 2016. Suramin treatment reduces chikungunya pathogenesis in mice. Antiviral Res 134:89–96.
Average Rating: 5 (Based on Reviews and 26 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















