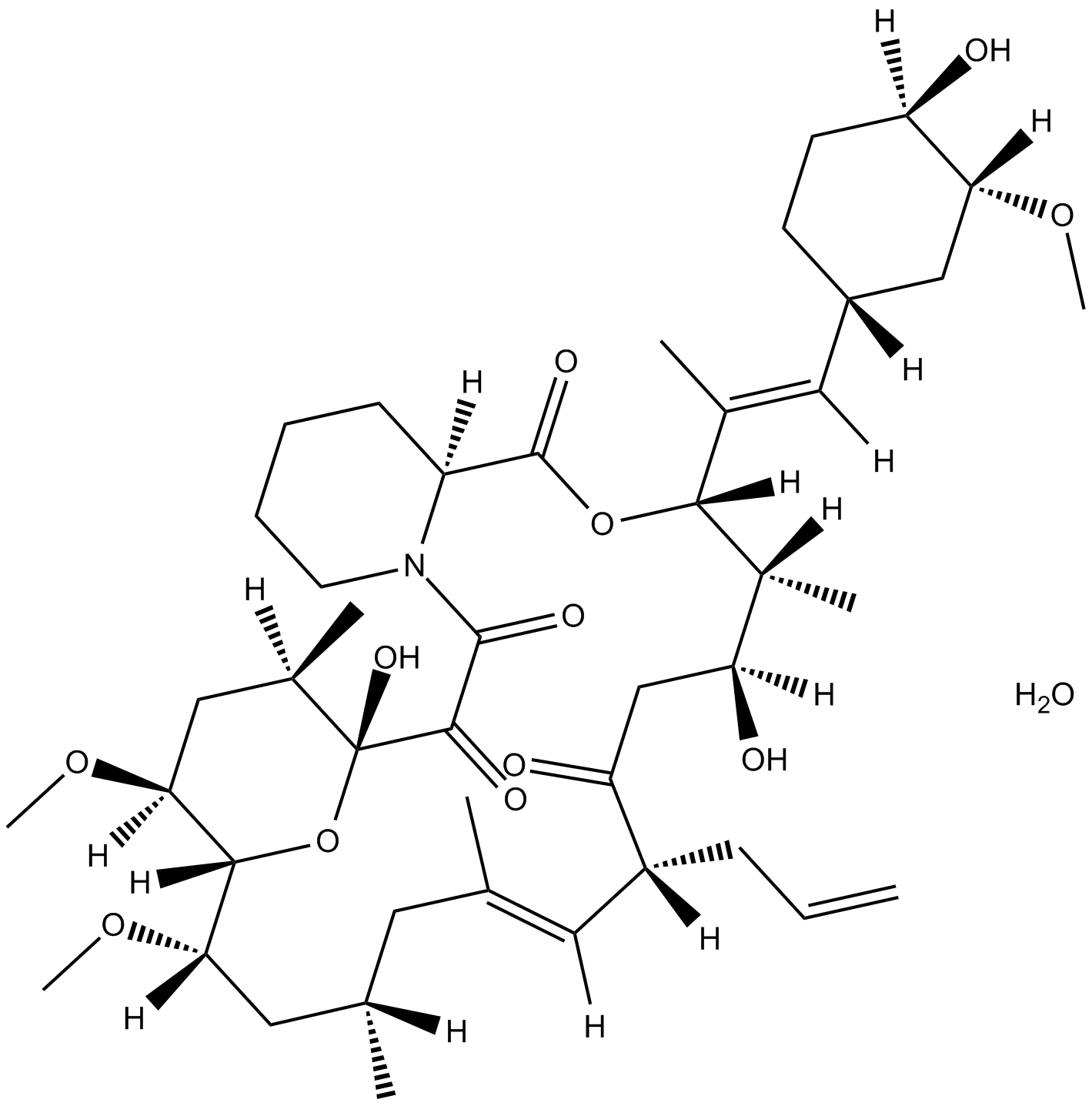
Tacrolimus monohydrate |
| Catalog No.GC17995 |
immunosuppressive drug
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 109581-93-3
Sample solution is provided at 25 µL, 10mM.
Tacrolimus monohydrate (FK506 monohydrate; Fujimycin monohydrate; FR900506 monohydrate) is a macrocyclic lactone with potent immunosuppressive properties, an immunosuppressant. Tacrolimus monohydrate binds to FK506 binding protein (FKBP) to form a complex and inhibits calcineurin phosphatase, which inhibits T-lymphocyte signal transduction and IL-2 transcription[1].
Tacrolimus monohydrate (FK506 monohydrate; Fujimycin monohydrate; FR900506 monohydrate) inhibits calcium-dependent events, such as IL-2 gene transcription, NO synthase activation, cell degranulation, and apoptosis. Tacrolimus also potentiates the actions of glucocorticoids and progesterone by binding to FKBPs contained within the hormone receptor complex, preventing degradation. The agent may enhance expression of the TGFβ-1 gene in a fashion analogous to that demonstrated for CsA. T cell proliferation in response to ligation of the T cell receptor is inhibited by Tacrolimus[1]. Treatment with a low concentration of Tacrolimus (FK506,10 μg/L) does not significantly affect the proliferation of MH3924A cells (P=0.135). Upon treatment with higher concentrations of Tacrolimus (100-1,000 μg/L), the proliferation of MH3924A cells is significantly enhanced (P0.05). However, when different concentrations of AMD3100 are combined with 100 μg/L Tacrolimus, the in vitro proliferation of MH3924A cells is increased (P<0.01)[3].
The therapeutic effect of Tacrolimus is investigated on progression and perpetuation of colitis by administering Tacrolimus to Dextran sulfate sodium (DSS)-treated mice from Days 10 to 16 or to 23. At Days 17 and 24, colon length is significantly shortened, and colon weight is significantly higher in DSS-treated control animals than in normal animals. In addition, colon weight per unit length in the control group is more than twice that in the normal group. While both 7 and 14 d treatment with Tacrolimus significantly suppresses increases in colon weight per unit length in DSS-treated animals compared with the control group, this treatment does not actually restore the colon shortening. In addition, this inhibitory effect of Tacrolimus on increases in colon weight per unit length is more pronounced with 14-d than 7-d treatment, as shown by the inhibitory percentages (59% vs. 28%)[4].
References:
[1]. Thomson AW, et al. Mode of action of Tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995 Dec;17(6):584-91.
[2]. Okada Y, et al. Tacrolimus ameliorates dextran sulfate sodium-induced colitis in mice: implication of interferon-γ and interleukin-1β suppression. Biol Pharm Bull. 2011;34(12):1823-7.
[3]. Vogel KR, et al. mTOR inhibitors rescue premature lethality and attenuate dysregulation of GABAergic/glutamatergic transcription in murine succinate semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. J Inherit Metab Dis. 2016 Nov;39(6):877-886.
[4]. Zhu H, et al. Tacrolimus promotes hepatocellular carcinoma and enhances CXCR4/SDF 1α expression in vivo. Mol Med Rep. 2014 Aug;10(2):585-92.
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















