Tetrabenazine (Synonyms: NSC 169886, NSC 172187, Ro 1-9569, TBZ) |
| Catalog No.GC13672 |
Tetrabenazine is the only US Food and Drug Administration-approved drug for Huntington's disease, indicated for treatment of chorea associated with Huntington's disease.
Products are for research use only. Not for human use. We do not sell to patients.
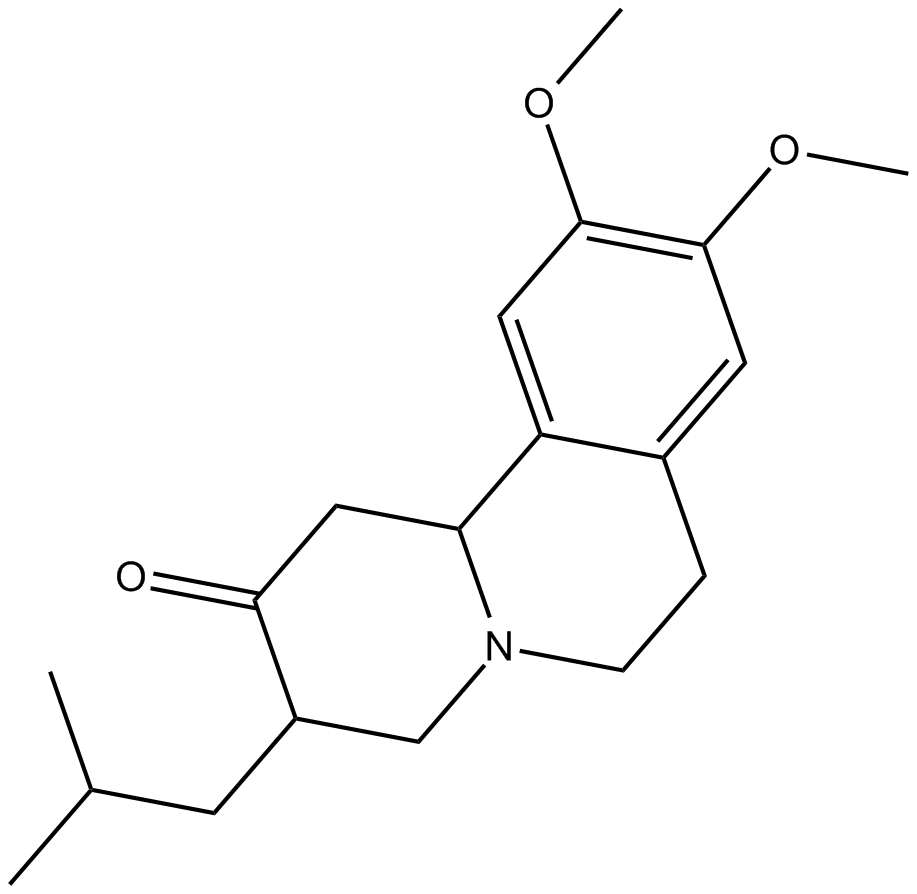
Cas No.: 58-46-8
Sample solution is provided at 25 µL, 10mM.
Tetrabenazine is the only US Food and Drug Administration-approved drug for Huntington's disease, indicated for treatment of chorea associated with Huntington's disease[1,3,4]. It reversibly inhibits central vesicular monoamine transporter (VMAT) transporter type 2 which acts on the various monoaminergic systems in the brain, e.g., dopamine, serotonin and noradrenaline[5] Tetrabenazine binds predominately to VMAT2 and has been shown to reversibly inhibit monoamine uptake in pre-synaptic vesicles, resulting in monoamine depletion of serotonin, dopamine, and nor-epinephrine [6] thereby reducing chorea[7].
In Neuro-2a neuroblastoma cell line, Tetrabenazine loaded nanoemulsion showed 100.00±1.23%, 100.00±2.01% and 100.00±2.09% cell viability when treated at the dose of 4.8ng/mL, 2.4ng/mL and 9.6ng/Ml[2]. When used bovine chromaffin cells (BCCs) challenged with repeated pulses of high K+ Upon repeated K+ pulsing, the exocytotic catecholamine release responses were gradually decaying. However, when cells were exposed to tetrabenazine, responses were mildly augmented and decay rate delayed[8].
In rat, The superiority of tetrabenazine nanoemulsion for delivering of tetrabenazine via intranasal route bypassing BBB[2]. In mice, Cold-water immersion-induced acute stress diminished the locomotor activity, exploratory behaviour, motor activity and social behaviour along with increase in the plasma corticosterone levels. Administration of tetrabenazine (1 and 2 mg/kg, i.p.), abolished the acute stress-induced behavioural and biochemical changes in a dose-dependent manner[9].
References:
[1]. Peter D, Vu T, et,al.Chimeric vesicular monoamine transporters identify structural domains that influence substrate affinity and sensitivity to tetrabenazine. J Biol Chem. 1996 Feb 9;271(6):2979-86. doi: 10.1074/jbc.271.6.2979. PMID: 8621690.
[2]. Arora A, Kumar S, et,al. Intranasal delivery of tetrabenazine nanoemulsion via olfactory region for better treatment of hyperkinetic movement associated with Huntington's disease: Pharmacokinetic and brain delivery study. Chem Phys Lipids. 2020 Aug;230:104917. doi: 10.1016/j.chemphyslip.2020.104917. Epub 2020 May 19. PMID: 32439327.
[3]. Kenney C, Hunter C, et,al. Short-term effects of tetrabenazine on chorea associated with Huntington's disease. Mov Disord. 2007 Jan;22(1):10-3. doi: 10.1002/mds.21161. PMID: 17078062.
[4]. Mestre TA, Ferreira JJ. An evidence-based approach in the treatment of Huntington's disease. Parkinsonism Relat Disord. 2012 May;18(4):316-20. doi: 10.1016/j.parkreldis.2011.10.021. Epub 2011 Dec 16. PMID: 22177624.
[5]. Thibaut F, Faucheux BA, et,al. Regional distribution of monoamine vesicular uptake sites in the mesencephalon of control subjects and patients with Parkinson's disease: a postmortem study using tritiated tetrabenazine. Brain Res. 1995 Sep 18;692(1-2):233-43. doi: 10.1016/0006-8993(95)00674-f. PMID: 8548309.
[6]. Pettibone DJ, Totaro JA, et,al. Tetrabenazine-induced depletion of brain monoamines: characterization and interaction with selected antidepressants. Eur J Pharmacol. 1984 Jul 20;102(3-4):425-30. doi: 10.1016/0014-2999(84)90562-4. PMID: 6489435.
[7]. Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006 Feb 14;66(3):366-72. doi: 10.1212/01.wnl.0000198586.85250.13. PMID: 16476934.
[8]. de Pascual R, Álvarez-Ortego N,et,al. Tetrabenazine Facilitates Exocytosis by Enhancing Calcium-Induced Calcium Release through Ryanodine Receptors. J Pharmacol Exp Ther. 2019 Oct;371(1):219-230. doi: 10.1124/jpet.119.256560. Epub 2019 Jun 17. PMID: 31209099.
[9].Kumar M, Singh N, et,al. Exploring the anti-stress effects of imatinib and tetrabenazine in cold-water immersion-induced acute stress in mice. Naunyn Schmiedebergs Arch Pharmacol. 2020 Sep;393(9):1625-1634. doi: 10.1007/s00210-020-01862-w. Epub 2020 Apr 14. PMID: 32291496.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















