Tigecycline (Synonyms: GAR 936, Glycylcycline) |
| Catalog No.GC14574 |
Glycylcycline antibiotic
Products are for research use only. Not for human use. We do not sell to patients.
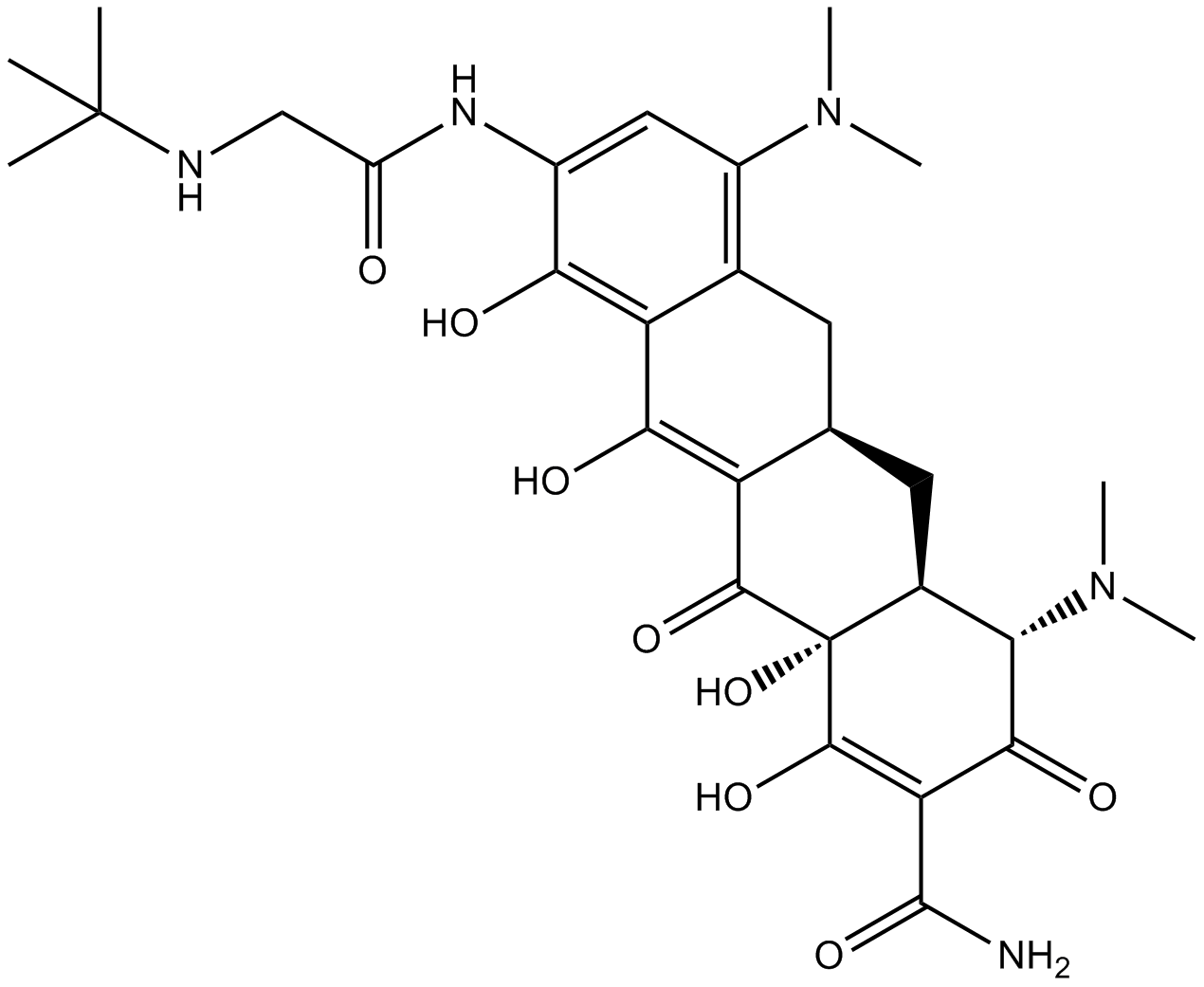
Cas No.: 220620-09-7
Sample solution is provided at 25 µL, 10mM.
Tigecycline, the first commercially available member of the glycylcyclines, is a new class of antimicrobial agents.
The glycylcyclines are the derivatives of tetracycline antibiotics, with modifications in the structure showing potent activity against gram-positive, gram-negative, and certain multidrug-resistant strains [1]. Tigecyclineis bacteriostatic could reversibly bind to the 30S ribosomal subunit thus inhibiting protein translation [1].
In vitro:Tigecycline exihibited good in vitro activities. The range of MIC90s was 0.12-0.5 μg/ml for vancomycin-susceptible and -resistant strains of Enterococcus faecalis and Enterococcus faecium [2]. Tigecyclinewas concentrated in cells and eliminated primarily via biliary excretion. Diminished renal function didn’t significantly alter its systemic clearance. Tigecycline didn’t interfere with common cytochrome P450 enzymes, making pharmacokinetic drug interactions uncommon [3].The tissue penetration of tigecycline was excellent and the compound showed equivalence to imipenem/cilastatin in intra-abdominal infection and to vancomycin plus aztreonam in skin and skin structure infection [4].
In vivo: In an intraperitoneal systemic murine infection model, tigecycline exihibited in vivo activities against GISA, methicillin-susceptible S. aureus and methicillin-resistant S. aureus strains [2]. Tigecycline and daptomycin showed similar in vivo efficacies against infections caused by the MSSA strain (strain GC 4543) with the ED50s of 0.12 and 0.24 mg/kg, respectively. The ED50s of tigecycline was 0.72 mg/kg [2].
Clinical trials: For complicated skin and skin-structure infections in hospitalized patients receiving tigecycline (50-mg, q12h), the microbial eradication rates and clinical cure rates were 70% and 74%. In patients who received 25-mg doses, the results were 56% and 67% [5]. Adverse events including increased nausea and vomiting appeared in treating patients with cSSSI. Tigecycline monotherapy was as safe and efficacious as the vancomycin-aztreonam combination in treating patients with cSSSI[6].
References:
[1] Rose W E, Rybak M J. Tigecycline: first of a new class of antimicrobial agents[J]. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 2006, 26(8): 1099-1110.
[2] Petersen P J, Bradford P A, Weiss W J, et al. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens[J]. Antimicrobial agents and chemotherapy, 2002, 46(8): 2595-2601.
[3] Stein G E, Craig W A. Tigecycline: a critical analysis[J]. Clinical infectious diseases, 2006, 43(4): 518-524.
[4] Livermore D M. Tigecycline: what is it, and where should it be used[J]. Journal of Antimicrobial Chemotherapy, 2005, 56(4): 611-614.
[5] Postier R G, Green S L, Klein S R, et al. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients[J]. Clinical therapeutics, 2004, 26(5): 704-714.
[6] Grosse E J E, Babinchak T, Dartois N, et al. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam[J]. Clinical infectious diseases, 2005, 41(Supplement 5): S341-S353.
Average Rating: 5 (Based on Reviews and 19 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















