Tigecycline mesylate |
| Catalog No.GC16441 |
First-in-class, broad spectrum antibiotic
Products are for research use only. Not for human use. We do not sell to patients.
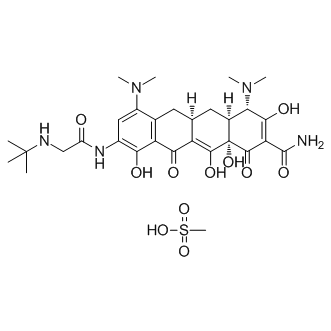
Cas No.: 1135871-27-0
Sample solution is provided at 25 µL, 10mM.
Tigecycline mesylate (GAR-936 mesylate) is a broad-spectrum glycylcycline antibiotic. The mean inhibitory concentration (MIC) of Tigecycline for E. coli (MG1655 strain) is approximately 125 ng/mL[1]. MIC50 and MIC90 are 1 and 2 mg/L for Acinetobacter baumannii (A. baumannii), respectively[2].
Tigecycline (0.63-30 µM, preincubated for 4 days, treated for 72 h) inhibits AML2 cells and HL-60 cells with IC50s of 4.72±0.54 and 3.06±0.85 μM (freshly prepared). Tigecycline inhibits AML2 cells and HL-60 cells with IC50s of 5.64±0.55 and 4.27±0.45 μM (1 day preincubation). Tigecycline inhibits AML2 cells and HL-60 cells with IC50s of 5.02±0.60 and 4.39±0.44 μM (2 day preincubation). Tigecycline inhibits AML2 cells and HL-60 cells with IC50s of 4.09±0.41 and 3.95±0.39 μM (3 day preincubation). After a 4 day preincubation of Tigecycline in saline, Tigecycline lost its ability to kill TEX human leukemia cells (from IC50~5 µM when freshly prepared to IC50>50 µM after 4 days preincubation) as measured by CellTiter Flour assay[1].
Tigecycline (50 mg/kg; intraperitoneal injection; twice a day; for 11 days) reduces tumor volume and weight in NOD/SCID mice[1]. The peak plasma concentration (Cmax), the terminal half-life (t1/2), area under the plasma concentration-time curve (AUC), clearance (CL) and volume of distribution (Vz) are 22.8μg/mL, 108.9 min, 1912.2min*μg/mL, 26.1 mL/min/kg, 4109.4 mL/kg for Tigecycline in saline, respectively. The peak plasma concentration (Cmax), the terminal half-life (t1/2), area under the plasma concentration-time curve (AUC), clearance (CL) and volume of distribution (Vz) are15.7μg/mL, 110.3 min, 2036.5 min*μg/mL, 24.6 mL/min/kg, 3906.2 mL/kg for Tigecycline in formulation (60 mg/mL pyruvate, 3 mg/mL ascorbic acid, pH 7 in saline) , respectively[1].
References:
[1]. Jitkova Y, et al. A novel formulation of tigecycline has enhanced stability and sustained antibacterial and antileukemic activity. PLoS One. 2014 May 28;9(5):e95281.
[2]. Falagas ME, et al. Activity of TP-6076 against carbapenem-resistant Acinetobacter baumannii isolates collected from inpatients in Greek hospitals. Int J Antimicrob Agents. 2018 Aug;52(2):269-271.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















