Z-Guggulsterone |
| Catalog No.GC10195 |
Z-Guggulsterone suppresses angiogenesis in vitro and in vivo with IC50 values of 1740, 1000, 220 and > 50000 nM for glucocorticoid, mineralocorticoid, androgen and farnesoid X receptors .
Products are for research use only. Not for human use. We do not sell to patients.
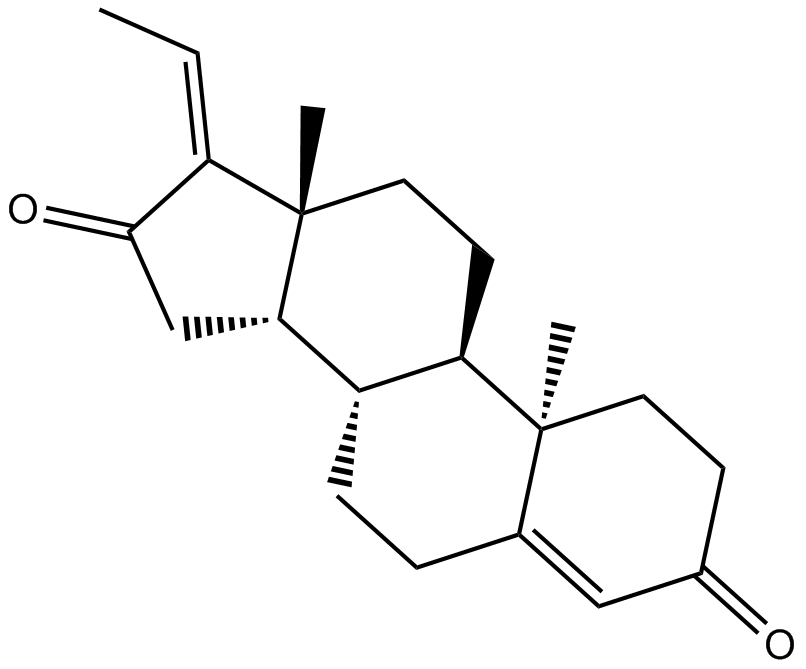
Cas No.: 39025-23-5
Sample solution is provided at 25 µL, 10mM.
Z-Guggulsterone, a component of the Ayurvedic medicinal plant Commiphora mukul, suppresses angiogenesis in vitro and in vivo with IC50 values of 1740, 1000, 220 and > 50000 nM for glucocorticoid, mineralocorticoid, androgen and farnesoid X receptors [1].
Bcl-2 protein expression was significantly decreased, and active caspase-3 and Bax protein expression was increased in SGC-7901 cells incubated with z-guggulsterone. The content of TNF-α was significantly increased, and the contents of VEGF and TGF-β1 were decreased in SGC-7901 cells incubated with z-guggulsterone[3]. In human umbilical vein endothelial cells (HUVEC) and DU145 cells, z-guggulsterone (5, 10 and 20 µM) significantly decrease cell migration in a concentration- and time-dependent manner, inhibiting capillary-like tube formation[4]. Z-guggulsterone (30 µM) simultaneously inhibited the expression of PXR and MDR1 at 24 h in human brain-derived microvessel endothelial cells (hBDMECs)[5].Z-guggulsterone attenuated TREM-1-mediated macrophage hyperactivation by suppressing TREM-1 expression and NF-κB and AP-1 activation[2].
Programmed death-ligand 1 (PD-L1) is an immune checkpoint molecule, that is overexpressed in non-small cell lung cancer (NSCLC) and has been associated with the response to anti-PD-1/PD-L1 immunotherapy.In vivo, Z-Guggulsterone treatment dose-dependently increased PD-L1 expression levels in mouse LLC tumor models[6]. Z-Guggulsterone significantly alleviated neurological deficits, infarct volume and histopathological damage in MCAO rats. Z-Guggulsterone successfully inhibited oxidative stress and inflammatory response in oxygen-glucose deprivation (OGD) treated neurons. Z-Guggulsterone exerted neuroprotective property through alleviated oxidative stress and inflammation via inhibiting the TXNIP/NLRP3 axis[7].
References:
[1]: Burris TP, Montrose C, et,al. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol. 2005 Mar;67(3):948-54. doi: 10.1124/mol.104.007054. Epub 2004 Dec 15. PMID: 15602004.
[2]: Che X, Park KC, et,al.Protective effects of guggulsterone against colitis are associated with the suppression of TREM-1 and modulation of macrophages. Am J Physiol Gastrointest Liver Physiol. 2018 Jul 1;315(1):G128-G139. doi: 10.1152/ajpgi.00027.2018. Epub 2018 Mar 15. PMID: 29543509.
[3]: Lv R, Zhu M, et,al. Z-Guggulsterone Induces Apoptosis in Gastric Cancer Cells through the Intrinsic Mitochondria-Dependent Pathway. ScientificWorldJournal. 2021 Jan 4;2021:3152304. doi: 10.1155/2021/3152304. PMID: 33488300; PMCID: PMC7801056.
[4]: Xiao D, Singh SV. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol Cancer Ther. 2008 Jan;7(1):171-80. doi: 10.1158/1535-7163.MCT-07-0491. PMID: 18202020.
[5]: Xu HB, Tang ZQ, et,al. Z-guggulsterone regulates MDR1 expression mainly through the pregnane X receptor-dependent manner in human brain microvessel endothelial cells. Eur J Pharmacol. 2020 May 5;874:173023. doi: 10.1016/j.ejphar.2020.173023. Epub 2020 Feb 19. PMID: 32087256.
[6]: Tian H, Gui Y, et,al. Z-guggulsterone induces PD-L1 upregulation partly mediated by FXR, Akt and Erk1/2 signaling pathways in non-small cell lung cancer. Int Immunopharmacol. 2021 Apr;93:107395. doi: 10.1016/j.intimp.2021.107395. Epub 2021 Jan 30. PMID: 33529916.
[7]: Liu T, Wang W, et,al. Z-Guggulsterone alleviated oxidative stress and inflammation through inhibiting the TXNIP/NLRP3 axis in ischemic stroke. Int Immunopharmacol. 2020 Dec;89(Pt B):107094. doi: 10.1016/j.intimp.2020.107094. Epub 2020 Oct 28. PMID: 33129097.
Average Rating: 5 (Based on Reviews and 17 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















