Z-VAD-FMK (Synonyms: Benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone,Z-Val-Ala-Asp(OMe)-FMK) |
| Catalog No.GC12861 |
Z-VAD-FMK (Z-Val-Ala-Asp(OMe)-FMK) is a cell-permeable and irreversible pan-caspase inhibitor. Z-VAD-FMK is an ubiquitin carboxy-terminal hydrolase L1 (UCHL1) inhibitor. Z-VAD-FMK irreversibly modifies UCHL1 by targeting the active site of UCHL1.
Products are for research use only. Not for human use. We do not sell to patients.
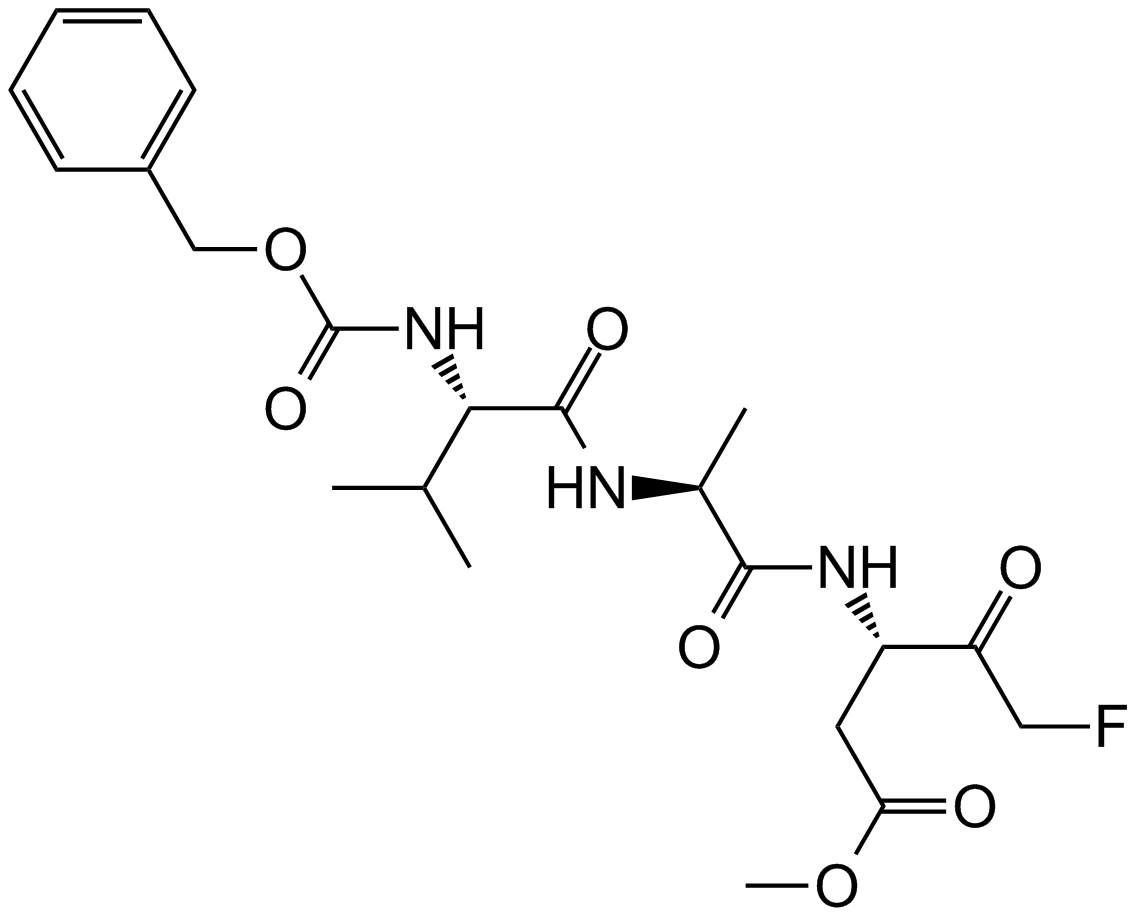
Cas No.: 187389-52-2
Sample solution is provided at 25 µL, 10mM.
Z-VAD-FMK (Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone), an ICE-like protease inhibitor, inhibits apoptosis by preventing the processing of CPP32 to its active form. [3]
Z-VAD-FMK is immunosuppressive in vitro and inhibit T cell proliferation without blocking the processing of caspase-8 and caspase-3. Z-VAD-FMK is capable of inhibiting T cell proliferation induced by anti-CD3 plus anti-CD28 or PHA. Besides, z-VAD-FMK inhibits caspase processing during apoptosis but not during T cell activation. Z-VAD-FMK Inhibits caspase processing and apoptosis induction in tumor cells in vitro (IC50 = 0.0015 - 5.8 mM). [1]
Z-VAD-FMK, can be used to induce necroptosis under certain stimuli. Treatment of mice with Z-VAD-FMK could significantly reduce mortality and alleviate disease after lipopolysaccharide (LPS) challenge. Notably, in LPS-challenged mice, treatment with Z-VAD-FMK could also reduce the percentage of peritoneal macrophages by promoting necroptosis and inhibiting pro-inflammatory responses in macrophages. What’s more, pretreatment with Z-VAD-FMK promoted LPS-induced nitric oxide-mediated necroptosis of bone marrow-derived macrophages (BMDMs), leading to reduced pro-inflammatory cytokine secretion. Interestingly, Z-VAD-FMK treatment promoted the accumulation of myeloid-derived suppressor cells (MDSCs) in a mouse model of endotoxin shock, and this process inhibited LPS-induced pro-inflammatory responses in macrophages. Treatment with Z-VAD-FMK alleviates LPS-induced endotoxic shock by inducing macrophage necroptosis and promoting MDSC-mediated inhibition of macrophage activation. For in vivo experienment, the mice were pretreated or post-treated with Z-VAD-FMK (5, 10, and 20 μg/g of body weight) or vehicle (saline) for 2 h and endotoxic shock was induced by an intraperitoneal injection of LPS (10 μg/g of body weight) and saline was used as control. [2]
References:
[1]. Lawrence CP, Chow SC. Suppression of human T cell proliferation by the caspase inhibitors, z-VAD-FMK and z-IETD-FMK is independent of their caspase inhibition properties. Toxicol Appl Pharmacol. 2012 Nov 15;265(1):103-12.
[2]. Li X, Yao X, Zhu Y, et al. The Caspase Inhibitor Z-VAD-FMK Alleviates Endotoxic Shock via Inducing Macrophages Necroptosis and Promoting MDSCs-Mediated Inhibition of Macrophages Activation. Front Immunol. 2019 Aug 2;10:1824.
[3]. Slee EA, Zhu H, et al. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996 Apr 1;315 (Pt 1) (Pt 1):21-4.
Average Rating: 5 (Based on Reviews and 34 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




