Ferrozine |
| Catalog No.GC47346 |
Ferrozina reacciona con hierro divalente para formar una especie compleja magenta estable y se utiliza para la determinación directa de hierro en agua con una absorbancia máxima a 562 nm.
Products are for research use only. Not for human use. We do not sell to patients.
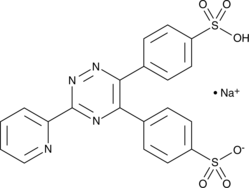
Cas No.: 69898-45-9
Sample solution is provided at 25 µL, 10mM.
Ferrozine reacts with divalent iron to form a stable magenta complex species and used for the direct determination of iron in water with maximum absorbance at 562 nm [1,2]. The visible absorption spectrum of the ferrous complex of ferrozine exhibits a single sharp peak at 562 nm. At this wavelength, the molar absorptivity is 27,900 and the Beer-ambert law is obeyed to approximately 4 mg/L of Fe [1].
Ferrozine assay of ISE6 cells[3]
The ferrozine assay for measuring non-haem iron was adapted to determine the concentration of iron in ISE6 cells. After knockdown and/or iron exposure, cells were collected, and cell lysates were collected using the method described above. Concentrated HCl (99.5%) was added and then heated to 95 °C. After cooling to room temperature, the mixture was centrifuged, and the supernatant was obtained, to which was added 75 mM ascorbate or water. Afterward, 10 mM ferrozine was added. Saturated ammonium acetate was added to facilitate colour development. Absorbance was measured at 550 nm, and the iron concentration was calculated based on a molar extinction coefficient of the iron-ferrozine complex of 27,900 M-1cm-1 and based on the protein concentration. The protein concentration was measured using a Micro BCA Protein Assay Kit. The total iron concentration is computed from samples with ascorbate. The ferrous iron concentration was computed from samples without ascorbate (reducing agent), while the ferric iron concentration is computed from the difference between the total iron and ferrous iron concentration.
References:
[1]. Stookey L L. Ferrozine---a new spectrophotometric reagent for iron[J]. Analytical chemistry, 1970, 42(7): 779-781.
[2]. Jeitner T M. Optimized ferrozine-based assay for dissolved iron[J]. Analytical biochemistry, 2014, 454: 36-37.
[3]. Hernandez E P, Kusakisako K, Talactac M R, et al. Induction of intracellular ferritin expression in embryo-derived Ixodes scapularis cell line (ISE6)[J]. Scientific reports, 2018, 8(1): 1-12.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















