AC 261066 |
| Katalog-Nr.GC11852 |
AC 261066 ist ein potenter, oral verfügbarer und Isoformen-selektiver Retinsäure-beta2 (RARbeta2)-Rezeptoragonist mit einem pEC50 von 8,0.
Products are for research use only. Not for human use. We do not sell to patients.
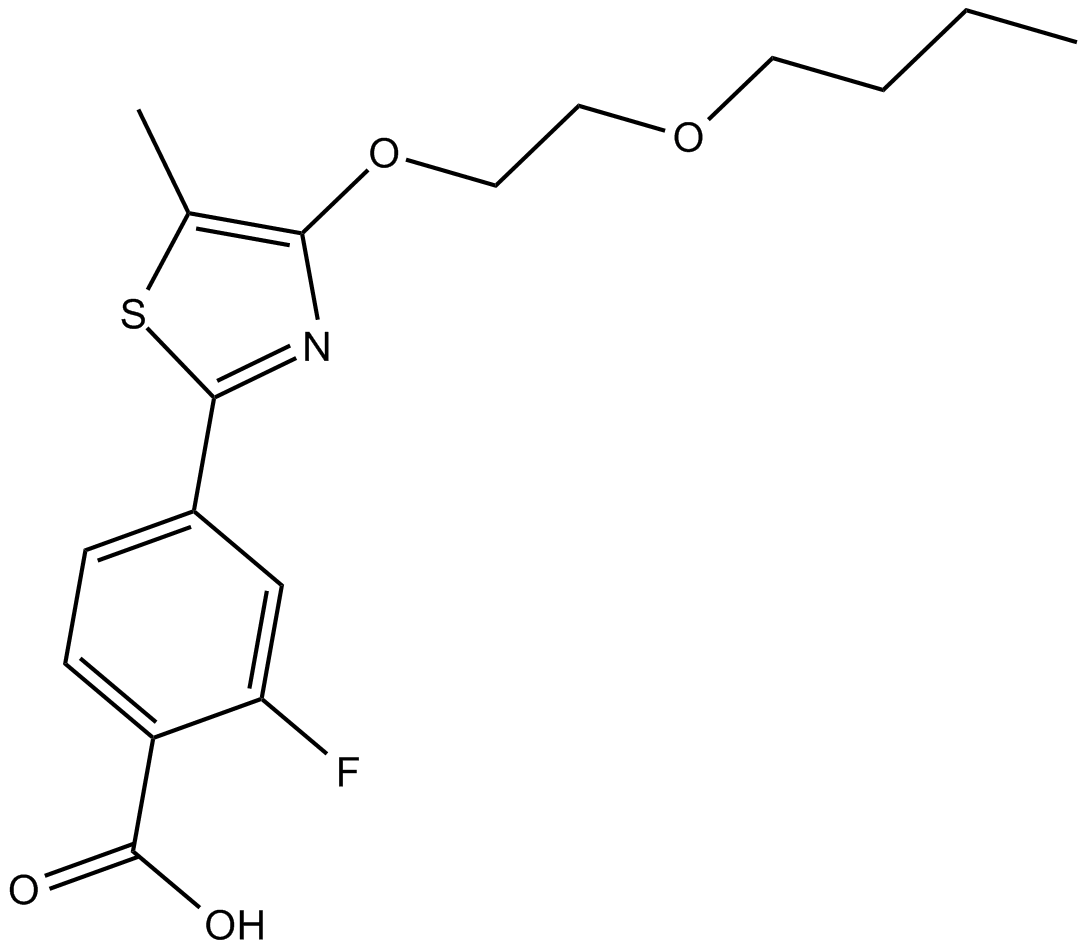
Cas No.: 870773-76-5
Sample solution is provided at 25 µL, 10mM.
AC 261066 is an agonist of retinoic acid receptor subtype β isoform 2 (RARβ2) [1] [2]. It is also called UVI2062 [3]. AC 261066 exhibited good oral bioavailability in rats (Foral= 52%) [4]. The IC50 of AC 261066 to AKR1B10 is 51 ± 7µM. No inhibition of AC 261066 to AKR1B1 was detected when using up to 100 µM AC 261066 in the enzymatic assay [3].
Retinoic acid receptors (RARs, subtypes α, β, γ) form heterodimeric complexes with retinoid X receptors (RXR α, β, γ) to orchestrate complex events, such as organ homoeostasis, immune function, development and reproduction [1]. RARβ2 is an isoform of subtype β of retinoic acid receptor, its affinity to retinoids and biological functions are different from other isoforms [2].
AC 261066 did not induce the cell adhesion of RPMI 8866 B cells to the ADAM28 disintegrin-like domain and did not block the atRA-induced adhesion [5].
AC 261066-treatment significantly inhibited the tail regeneration process in N. viridescens compared to DMSO controls. When these AC 261066-treated newts were removed from AC 261066 at 21 days (time of analysis) and then placed back into normal pond water, tail regeneration commenced. Five weeks after removal from the AC 26l066, the tail had regenerated, albeit with an alteration in the dorsal-ventral patterning compared to a normal tail regenerate [6].
References:
[1]. Albane le Maire, Susana Álvarez, Pattabhiraman Shankaranarayanan, et al. Retinoid Receptors and Therapeutic Applications of RAR/RXR Modulators. Current Topics in Medicinal Chemistry, 2012, 12: 505-527.
[2]. Christopher R. Gardner, Belamy B. Cheung, Jessica Koach, et al. Synthesis of retinoid enhancers based on 2-aminobenzothiazoles for anti-cancer therapy. Bioorg. Med. Chem., 2012, 20: 6877-6884.
[3]. Sergio Porté, Joan Giménez, Marta Dominguez, et al. Aldo–keto reductases in retinoid metabolism: Search for substrate specificity and inhibitor selectivity. Chemico-Biological Interactions, 2013, 202(1-3):186-94.
[4]. Birgitte W. Lund, Fabrice Piu, Natalie K. Gauthier, et al. Discovery of a Potent, Orally Available, and Isoform-Selective Retinoic Acid β2 Receptor Agonist. J. Med. Chem., 2005, 48: 7517-7519.
[5]. Jarrett T. Whelan, Lei Wang, Jianming Chen, et al. Retinoids induce integrin-independent lymphocyte adhesion through RAR-α nuclear receptor activity. Biochemical and Biophysical Research Communications, 2014, 454: 537-542.
[6]. Christopher J. Carter. Identification of novel retinoid receptors and their roles in vertebrate and invertebrate nervous systems [D]. St. Catharines: Brock University, 2011.
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















