LL-37 (trifluoroacetate salt) (Synonyms: CAP-18, hCAP-18, Cathelicidin, FALL-39) |
| Catalog No.GC14377 |
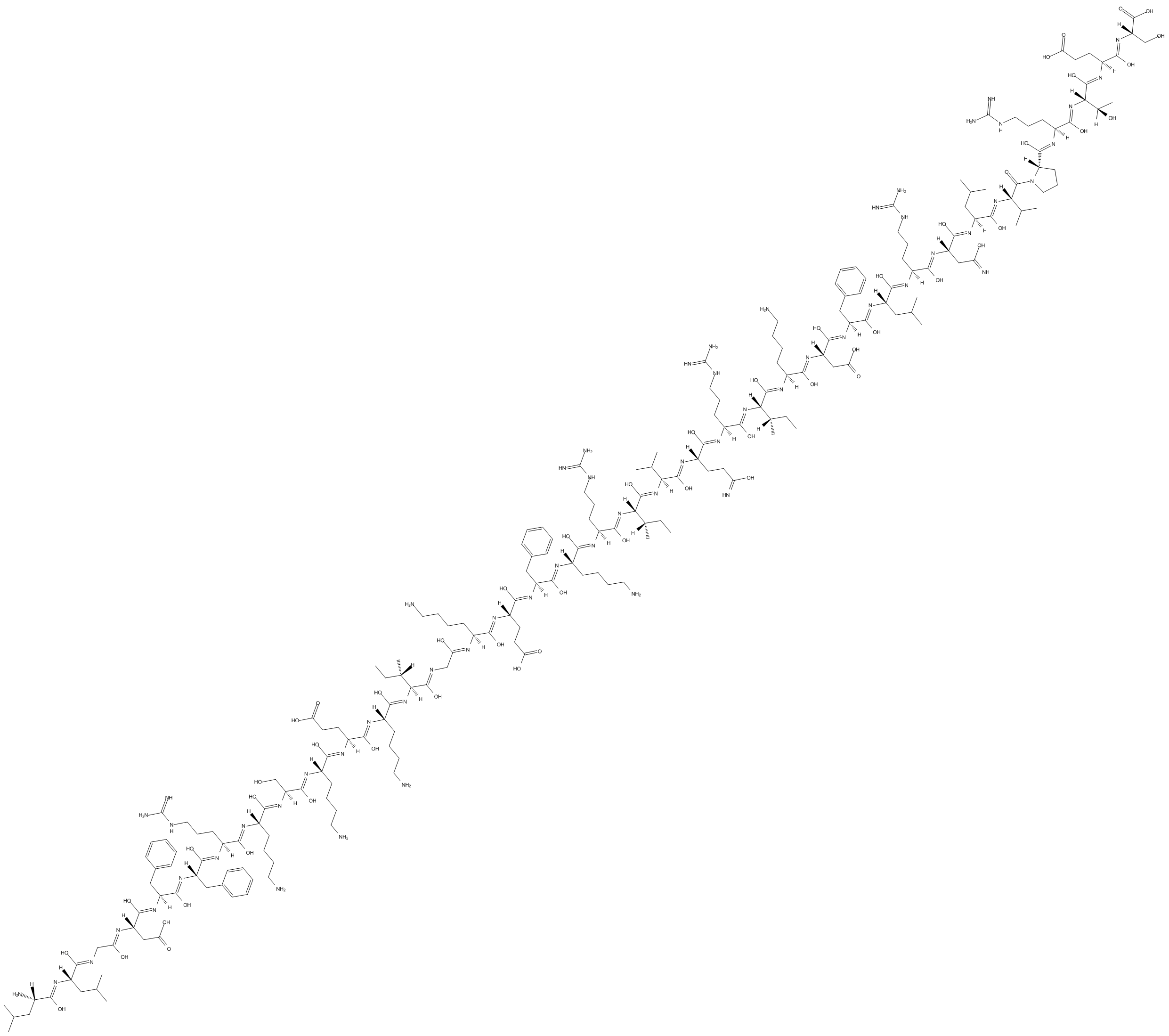
LL-37 (trifluoroacetate salt) is a 37-residue, amphipathic, cathelicidin-derived antimicrobial peptide, which exhibits a broad spectrum of antimicrobial activity.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 154947-66-7
Sample solution is provided at 25 µL, 10mM.
LL-37 contains 37 amino acid residues with the first two leucine residues (L1LGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES37) and mostly exists in epithelial cells and neutrophils.
In vitro experiment it shown that treatment with 4 and 10 μM LL-37 reduced both the number and viability of human osteoblast-like MG63 cell.[2] In vitro, the pretreatment of pMSCs with 1 and 10 μg/mL of LL-37 had not effect on the migratory cells ability. But the pretreatment with 1 μg/mL of LL-37 increased the migratory potential of pMSCs after 48 h.[4] In vitro study it indicated that at 1 µmol/L concentrations of LL-37 for 24 hours, LL-37 prevented LPS-induced stimulation of MCP-1 expression analyzed both on transcript and on protein levels, but had no effect on toll-like receptor (TLR)2 and TLR4 transcript expression. In the meanwhile, treatment with 0.1 and 1 µmol/L LL-37 for 60 minutes in PDL cell induced immunoreactivity for LL-37.[5]
In vivo study it suggested that treatment with 2 μg/mouse of LL-37 intravenously improves the survival of CLP septic mice in a dose-dependent effect. LL-37 ameliorates the level of ectosomes with higher antibacterial potential, result in reducing the bacterial load in CLP mice.[1] LL-37 dose-dependently (1, 3, or 10 μg/ml) induced ectosome release from neutrophil. Injection LL-37 supressed the infiltration of polymorphonuclear cells in CLP mice, where the bacterial burden and inflammatory response are decreased.[3]
References:
[1].Nagaoka I, et al. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int J Mol Sci. 2020 Aug 19;21(17):5973.
[2].Bankell E, et al. LL-37-induced caspase-independent apoptosis is associated with plasma membrane permeabilization in human osteoblast-like cells. Peptides. 2021 Jan;135:170432.
[3].Kumagai Y, et al. Antimicrobial peptide LL-37 ameliorates a murine sepsis model via the induction of microvesicle release from neutrophils. Innate Immun. 2020 Oct;26(7):565-579.
[4].Oliveira-Bravo M, et al. LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells. Stem Cell Res Ther. 2016 Dec 30;7(1):189.
[5].Aidoukovitch A, et al. The host defense peptide LL-37 is internalized by human periodontal ligament cells and prevents LPS-induced MCP-1 production. J Periodontal Res. 2019 Dec;54(6):662-670.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















