4-Octyl Itaconate |
| Catalog No.GC31648 |
4-Octyl Itaconate?(4-OI) is a cell-permeable itaconate derivative. Itaconate and 4-Octyl Itaconate?had similar thiol reactivity, making 4-Octyl Itaconate?a suitable itaconate surrogate to study its biological function.
Products are for research use only. Not for human use. We do not sell to patients.
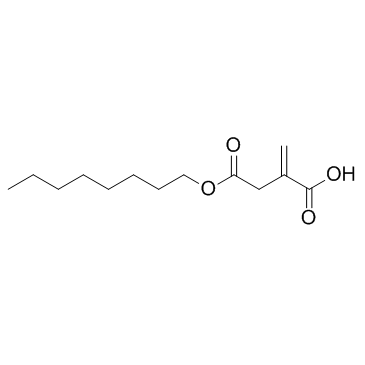
Cas No.: 3133-16-2
Sample solution is provided at 25 µL, 10mM.
4-Octyl Itaconate (4-OI) is a cell-permeable itaconate derivative. Itaconate and 4-Octyl Itaconate had similar thiol reactivity, making 4-Octyl Itaconate a suitable itaconate surrogate to study its biological function. 4-Octyl Itaconate is reported to alkylate cysteine residues on kelchlike ECH-associated protein 1 (KEAP1) and then activate nuclear factor (erythroid derived 2)-related factor 2 (Nrf2) to exert antioxidant and anti-inflammatory effects. [1]. 4-Octyl Itaconate can halt the progress of various diseases, including ischemiareperfusion injury, osteoclast-related diseases, and renal fibrosis, by reducing oxidative stress and modifying the immune response of macrophages to lipopolysaccharide (LPS) [2].
The inhibitory effect of 4-octyl itaconate extends to the replication of several other pathogenic viruses including Herpes Simplex Virus-1 and-2, Vaccinia virus, and Zika virus through a type I interferon (IFN)- independent mechanism. In addition, 4-Octyl Itaconate limit host inflammatory responses to SARS-CoV2 infection [3].
Treat Vero cells with 4-octyl itaconate enerated before infection with SARS-CoV2 resulted in a 102–104 eduction in SARS-CoV2 RNA levels in a dose dependent manner, while not affecting cell viability, as determined by lactate dehydrogenase (LDH) release assay. The antiviral effect of 4-Octyl Itaconate was also demonstrated in the lung cancer cell line Calu-3, where SARS-CoV2 RNA levels were reduced by >2-logs, while release of progeny virus was reduced by >6-logs. 4-Octyl Itaconate effect SARSCoV2 in primary human airway epithelial (HAE) cultures, also, 4-Octyl Itaconate treatment significantly reduced viral RNA levels. The antiviral effect of 4-Octyl Itaconate was reproduced using a different SARS-CoV-2 isolate [3].
4-Octyl Itaconate (50 mg/ kg) were administered to 57BL/6J mice or vehicle 2 h before intraperitoneal injection of LPS (5 mg/kg). 4-Octyl Itaconate treatment significantly prolonged the survival rate and simultaneously decreased the serum levels of IL-1β, IL-6 and lactate in mice induced by LPS. 4-Octyl Itaconate protects mice against experimental lethal endotoxaemia partly by inhibiting cytokine release and lactate production [1].
References:
[1] Liao, S. T. et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat. Commun. 10, 5091, 2019.
[2] Li, Y., Chen, X., Zhang, H., et al. 4-Octyl Itaconate Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting Oxidative Stress and Inflammation. Dddt 14, 5547–5558. 2020. doi:10.2147/DDDT.S280922.
[3] Olagnier, D. et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 11, 4938, 2020.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















