AZD8055 (Synonyms: CCG-168) |
| Catalog No.GC16380 |
Inhibiteur de MTOR
Products are for research use only. Not for human use. We do not sell to patients.
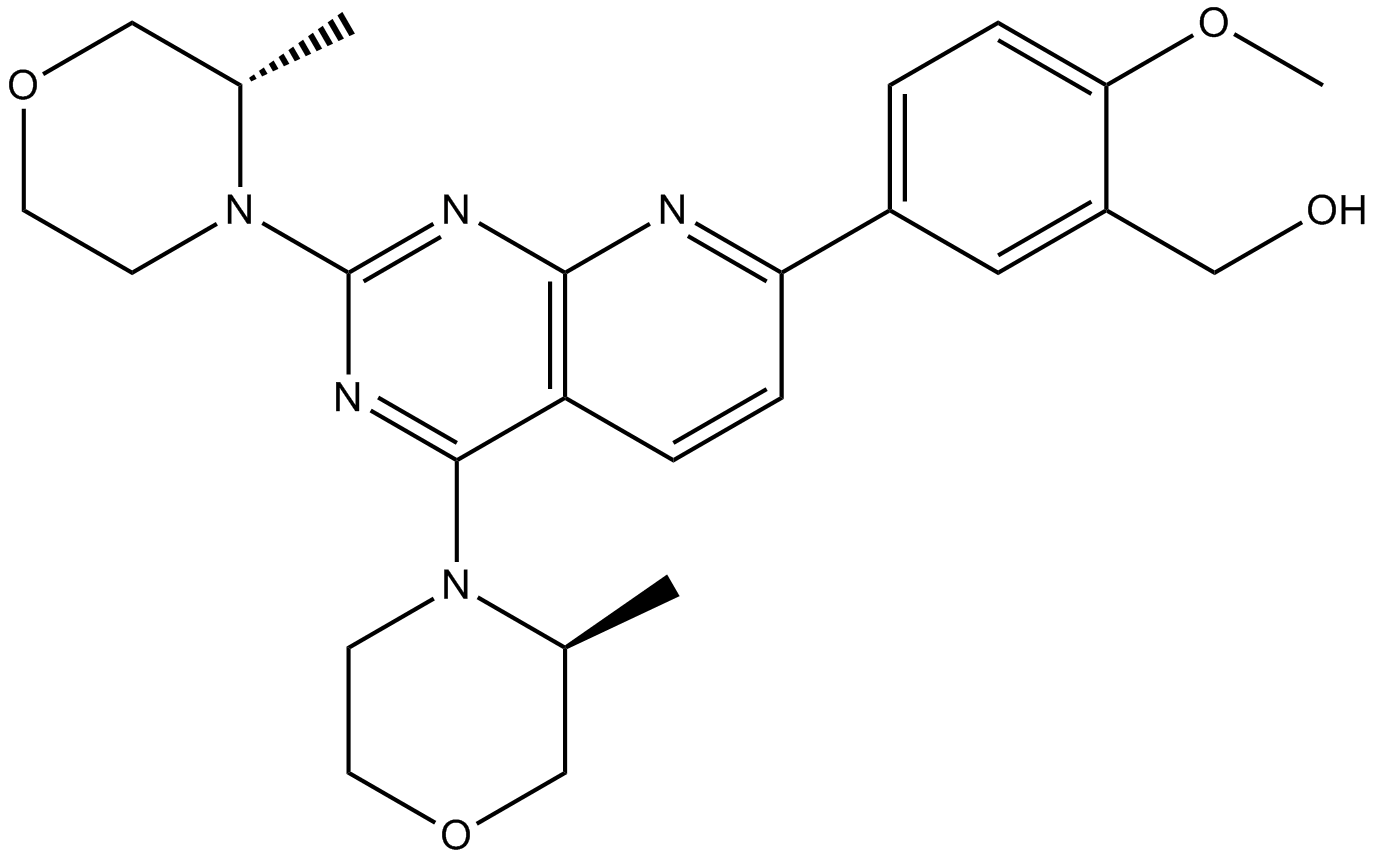
Cas No.: 1009298-09-2
Sample solution is provided at 25 µL, 10mM.
AZD8055 is a selective inhibitor of mTOR kinase with IC50 of 0.8 nM [1]. It competes with ATP at the ATP-binding cleft of mTOR. AZD8055 showed ~1000 fold selectivity against closely related kinases PI3K isoforms and ATM/DNA-PK [1]. Furthermore, it had no significant activity against a panel of 260 kinases at 10 μM [1].
AZD8055 has shown potential anti-tumor activity in preclinical studies.
mTOR phosphorylates and activates transcription factors which in turn regulate cell growth, proliferation, motility, and survival. AZD8055 inhibited downstream signaling of both mTORC1 and mTORC2 complexes [1]. It inhibited proliferation in cell lines of lung, cervical and laryngeal cancer, as well as acute myeloid leukemia [1-4]. Studies in xenograft models showed that AZD8055 reduced tumor growth of glioblastoma, breast, lung, colon, prostate, uterine, and head and neck cancer [1, 5]. In addition, AZD8055 enhanced the efficacy of HDAC inhibitors and MEK inhibitors [6-8]. AZD8055 has been tested in phase I clinical trials with minimal clinical benefit [9, 10].
References:
[1]Chresta CM, Davies BR, Hickson I et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 2010; 70: 288-298.
[2]Li S, Li Y, Hu R et al. The mTOR inhibitor AZD8055 inhibits proliferation and glycolysis in cervical cancer cells. Oncol Lett 2013; 5: 717-721.
[3]Willems L, Chapuis N, Puissant A et al. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia 2012; 26: 1195-1202.
[4]Zhao L, Teng B, Wen L et al. mTOR inhibitor AZD8055 inhibits proliferation and induces apoptosis in laryngeal carcinoma. Int J Clin Exp Med 2014; 7: 337-347.
[5]Li Q, Song XM, Ji YY et al. The dual mTORC1 and mTORC2 inhibitor AZD8055 inhibits head and neck squamous cell carcinoma cell growth in vivo and in vitro. Biochem Biophys Res Commun 2013; 440: 701-706.
[6]Shao H, Gao C, Tang H et al. Dual targeting of mTORC1/C2 complexes enhances histone deacetylase inhibitor-mediated anti-tumor efficacy in primary HCC cancer in vitro and in vivo. J Hepatol 2012; 56: 176-183.
[7]Holt SV, Logie A, Davies BR et al. Enhanced apoptosis and tumor growth suppression elicited by combination of MEK (selumetinib) and mTOR kinase inhibitors (AZD8055). Cancer Res 2012; 72: 1804-1813.
[8]Renshaw J, Taylor KR, Bishop R et al. Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin Cancer Res 2013; 19: 5940-5951.
[9]Asahina H, Nokihara H, Yamamoto N et al. Safety and tolerability of AZD8055 in Japanese patients with advanced solid tumors; a dose-finding phase I study. Invest New Drugs 2013; 31: 677-684.
[10]Naing A, Aghajanian C, Raymond E et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer 2012; 107: 1093-1099.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















