Oxonic acid potassium salt (Synonyms: Allantoxanic Acid, Potassium oxonate) |
| Catalog No.GC17025 |
Oxonic acid potassium salt is an inhibitor of uricase, inhibits the phosphorylation of 5-FU to 5-fluorouridine-5'-monophosphate catalyzed by pyrimidine phosphoribosyl-transferase in a different manner from allopurinol in cell-free extracts and intact cells in vitro.
Products are for research use only. Not for human use. We do not sell to patients.
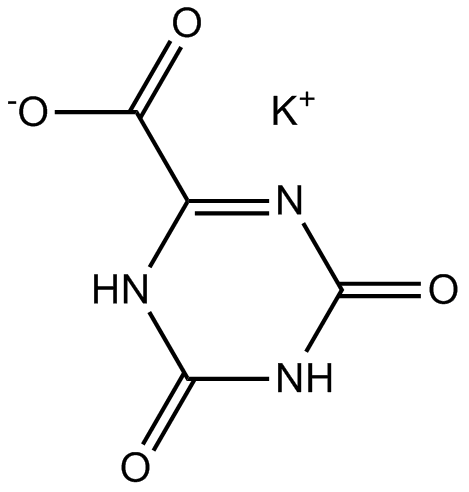
Cas No.: 2207-75-2
Sample solution is provided at 25 µL, 10mM.
Potassium oxonate is an inhibitor of uricase, inhibits the phosphorylation of 5-FU to 5-fluorouridine-5'-monophosphate catalyzed by pyrimidine phosphoribosyl-transferase in a different manner from allopurinol in cell-free extracts and intact cells in vitro.IC50 value: Target: On p.o. administration of 5-FU (2 mg/kg) and a potent inhibitor of 5-FU degradation to Yoshida sarcoma-bearing rats, oxonic acid (10 mg/kg) was found to inhibit the formation of 5-fluorouridine-5'-monophosphate from 5-FU and its subsequent incorporation into the RNA fractions of small and large intestine but not of tumor and bone marrow tissues [1]. Oxonic acid diet increased plasma uric acid by 80-90 micromol/l, while blood pressure was elevated only in hyperuricemic 5/6 nephrectomy rats (18 mmHg) [2].
References:
[1]. Shirasaka T, et al. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993 Sep 1;53(17):4004-9.
[2]. Eraranta A, et al. Oxonic acid-induced hyperuricemia elevates plasma aldosterone in experimental renal insufficiency. J Hypertens. 2008 Aug;26(8):1661-8.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















