Sulforaphane (Synonyms: SFN) |
| Catalog No.GC14016 |
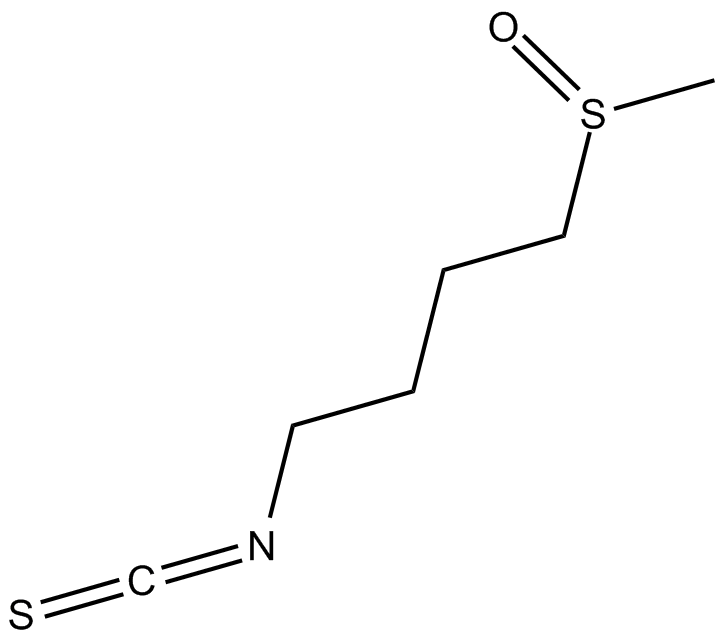
Sulforaphane (SFN) known as [1-isothiocyanato-4-(methylsulfinyl)butane].
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 4478-93-7
Sample solution is provided at 25 µL, 10mM.
Sulforaphane (SFN) known as [1-isothiocyanato-4-(methylsulfinyl)butane]. It is an isothiocyanate that is present naturally in widely consumed vegetables and has a particularly high concentration in broccoli[1,10]. Sulforaphane is a phytocompound with antioxidant, anti-inflammatory, and antiapoptotic effects[2].
Sulforaphane amplifies ALDH2 activity in HepaRG cells and suppresses acetaldehyde-induced proliferation and activation in LX-2 cells[3]. Sulforaphane reversed mesenchymal-like changes induced by TGF-β1 and restored cells to their epithelial-like morphology. The expression of the epithelial marker, E-cadherin, increased after Sulforaphane treatment, while expression of the mesenchymal markers, N-cadherin, vimentin, and α-SMA decreased in A549 cells after SFN treatment[4]. Sulforaphane inhibited cell growth and reduced collagen at the mRNA and protein levels in keloid fibroblasts. Moreover, sulforaphane markedly suppressed the expression of IL-6 and α-SMA and inhibited Stat3 and Smad3 signaling pathways in keloid fibroblast KF112 cells[8]. Sulforaphane inhibited XWLC-05 cell growth with inhibitory concentration (IC)50 of 4.04, 3.38, and 3.02 µg/mL at 24, 48, and 72 hours, respectively. Sulforaphane affected the XWLC-05 cell cycle as cells accumulated in the G2/M phase[9].
Sulforaphane exerts antioxidative effects with ALDH2 induction in liver fibrosis induced by ethanol exposure in CCl4-treated mice[3]. Nrf2 plays the indispensable role for Sulforaphane cardiac protection from T2DM with significant induction of MT and other antioxidants. MT expression induced by Sulforaphane is Nrf2 dependent, but is not indispensable for SFN-induced cardiac protection from T2DM[5]. Sulforaphane significantly reduced the incidence and size of 4NQO-induced tongue tumors in mice[6]. Sulforaphane suppresses skin cancer via blocking sulfatase-2 with subsequent elevation in HSPGs and reduction in glypican-3. Moreover, sulforaphane attenuated skin cancer-induced activation of inflammatory and apoptotic pathways[7].
References:
[1]. Panjwani AA, Liu H, et,al. Crucifers and related vegetables and supplements for neurologic disorders: what is the evidence? Curr Opin Clin Nutr Metab Care. 2018 Nov;21(6):451-457. doi: 10.1097/MCO.0000000000000511. PMID: 30199394.
[2]. Schepici G, Bramanti P, et,al. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int J Mol Sci. 2020 Nov 16;21(22):8637. doi: 10.3390/ijms21228637. PMID: 33207780; PMCID: PMC7698208.
[3]. Ishida K, Kaji K, et,al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem. 2021 Mar;89:108573. doi: 10.1016/j.jnutbio.2020.108573. Epub 2020 Dec 31. PMID: 33388347.
[4]. Kyung SY, Kim DY, et,al. Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition. BMC Pharmacol Toxicol. 2018 Apr 2;19(1):13. doi: 10.1186/s40360-018-0204-7. PMID: 29609658; PMCID: PMC5879815.
[5]. Gu J, Cheng Y, et,al. Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy. Diabetes. 2017 Feb;66(2):529-542. doi: 10.2337/db15-1274. Epub 2016 Nov 30. PMID: 27903744; PMCID: PMC5248986.
[6]. Bauman JE, Zang Y, et,al. Prevention of Carcinogen-Induced Oral Cancer by Sulforaphane. Cancer Prev Res (Phila). 2016 Jul;9(7):547-57. doi: 10.1158/1940-6207.CAPR-15-0290. Epub 2016 Jun 23. PMID: 27339168; PMCID: PMC4930727.
[7]. Alyoussef A, Taha M. Antitumor activity of sulforaphane in mice model of skin cancer via blocking sulfatase-2. Exp Dermatol. 2019 Jan;28(1):28-34. doi: 10.1111/exd.13802. Epub 2018 Dec 11. PMID: 30315662.
[8]. Kawarazaki A, Horinaka M, et,al. Sulforaphane suppresses cell growth and collagen expression of keloid fibroblasts. Wound Repair Regen. 2017 Apr;25(2):224-233. doi: 10.1111/wrr.12512. Epub 2017 Feb 20. PMID: 28120534.
[9]. Zhou L, Yao Q, et,al. Sulforaphane-induced apoptosis in Xuanwei lung adenocarcinoma cell line XWLC-05. Thorac Cancer. 2017 Jan;8(1):16-25. doi: 10.1111/1759-7714.12396. Epub 2016 Nov 23. PMID: 27878984; PMCID: PMC5217876.
[10].Gamet-Payrastre L, Li P, et,al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000 Mar 1;60(5):1426-33. PMID: 10728709.
Average Rating: 5 (Based on Reviews and 23 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




