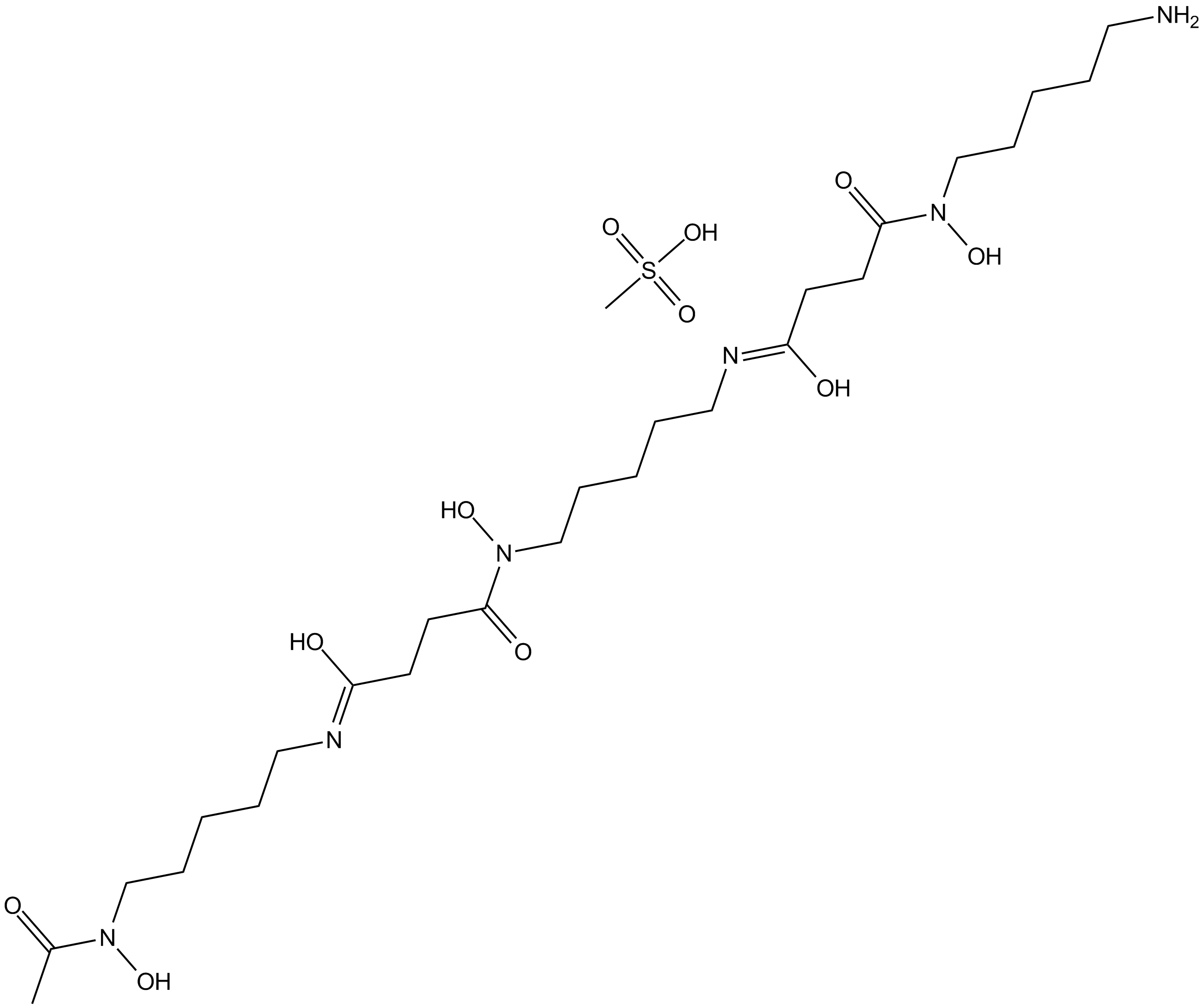
Deferoxamine Mesylate
In the vast expanse of medical science, where each discovery is a step towards unraveling the mysteries of human health, Deferoxamine Mesylate emerges as a pivotal breakthrough in the realm of iron chelation therapy. This remarkable compound, often cloaked in its medical terminology, is a beacon of hope for individuals grappling with conditions of iron overload, a perilous state where excess iron in the body heralds a suite of complications.
Iron, an essential element for life, plays a critical role in various physiological processes, including oxygen transport and DNA synthesis. However, akin to a double-edged sword, its excess can unleash oxidative stress, damaging cells and organs. Herein lies the significance of Deferoxamine Mesylate – a master key designed to unlock the chains of excess iron, safeguarding the body's delicate balance.
Molecular Formula: C26H52N6O11S
Molecular Weight: 656.8 g/mol
Synonyms: Deferoxamine mesilate
Desferal
Deferoxamine B mesylate
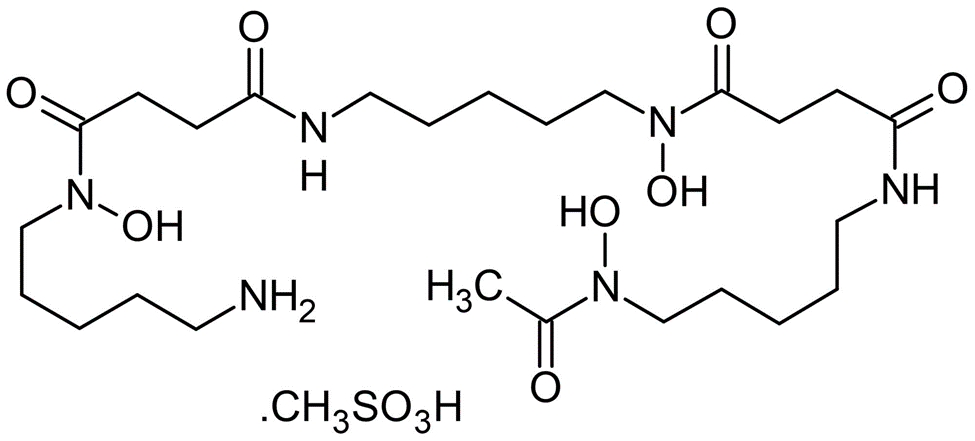
Deferoxamine Mesylate is a natural product isolated from Streptomyces Pilosus. It forms iron complex and is used as a chelating agent, particularly in mesylate form. It is the mesylate salt of an iron chelating agent that binds free iron in a stable complex, preventing it from engaging in chemical reactions. This compound chelates iron from intra lysosomal ferritin and ferrioxamine, a water soluble complex excreted by the kidneys and in the faeces via the bile. This agent does not readily chelate iron bound to transferrin, hemoglobin, myoglobin or cytochrome.
As per our knowledge it is known that vitamin E has potent iron chelation properties. There-fore, we sought to examine whether the protective effect of alpha-tocotrienol against glutamate-induced loss of viability was mediated by the metal chelation property of vitamin E. Gluta-mate-induced death of HT4 cells was partially inhibited by the iron chelator deferoxamine mesylate. The iron chelator regulated a late event in the death pathway because significant protection was achieved even when cells were treated with deferoxamine mesylate 4 h after glutamate treatment.
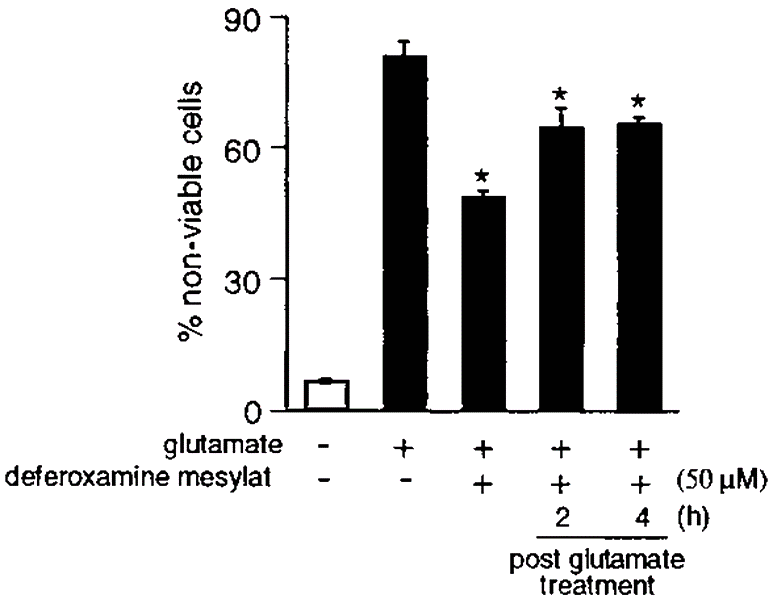
Figure 1: Deferoxamine mesylate partially protects HT4 cells
Deferoxamine mesylate partially protects HT4 cells against glutamate-induced death. Cells were treated with the iron chelator deferoxamine mesylate either 5 min before or after glutamate treatment as indicated in the figure. *, p 0.05 when compared with desferoxamine mesylate non treated and glutamate treated groups.
Clinical studies and patient outcomes have consistently highlighted the efficacy of deferoxamine mesylate in managing iron levels. Its role in preventing and treating iron-induced heart disease, liver damage, and endocrine disorders is well documented. However, the drug's administration through subcutaneous or intravenous infusion can be a significant drawback for some patients, leading to the exploration of alternative iron chelators that can be taken orally.
Despite these challenges, the advent of deferoxamine mesylate has been a game-changer in the field of hematology. It has not only extended the lifespan of patients with conditions like thalassemia major but has also improved their quality of life significantly. The drug's ability to mitigate the risks associated with iron overload, such as cardiac complications, liver diseases, and diabetes, underscores its importance in therapeutic regimens.
Deferoxamine Mesylate is also have a role in up regulation of LC3-II expression. To dissect the mechanisms involved in the up-regulation of LC3-II expression observed with Dp44mT and the potential roles of iron chelation in this process, studies were initiated to compare the effects of DFO to Dp44mT. In all studies, we use DFO at 250 μm due to its lower permeability and iron chelation efficacy ,whereas Dp44mT was examined at 5 μm.
DFO significantly (p < 0.01) increased NDRG1 expression (upper, ∼44-kDa band only) in PANC1 VC cells relative to the control (Fig A). As evident after incubation of PANC1 N1 cells with Dp44mT, DFO had no significant (p > 0.05) effect on NDRG1 expression due to the marked exogenous NDRG1 levels at 45 kDa (Fig A). In both PANC1 VC and N1 cells, Dp2mT (5 μm) had no significant (p > 0.05) effect on NDRG1 expression relative to their respective controls (Fig2 A). Examining LC3-II expression in PANC1 VC cells, both Dp44mT and DFO significantly (p < 0.01) increased LC3-II levels, although Dp44mT was significantly (p < 0.01) more effective than DFO. In contrast, Dp2mT had no significant (p > 0.05) effect on LC3-II expression in PANC1 VC cells (Fig 2A). NDRG1 overexpression significantly (p < 0.05) suppressed the increase in LC3-II levels mediated by Dp44mT or DFO, although again, Dp2mT had no significant effect on LC3-II expression (Fig2 A and B). Collectively, considering that Dp2mT cannot bind cellular iron and that DFO binds cellular iron without ROS generation ,these studies demonstrated that iron deprivation was involved in increasing LC3-II expression after incubation with chelators. These results were confirmed using immunofluorescence studies where 24 h of incubation with either Dp44mT or DFO increased LC3-II punctate staining in cells relative to the control in both PANC1 VC and PANC1 N1 cells, although again, NDRG1 suppressed LC3-II expression with both chelators (Fig 2C).
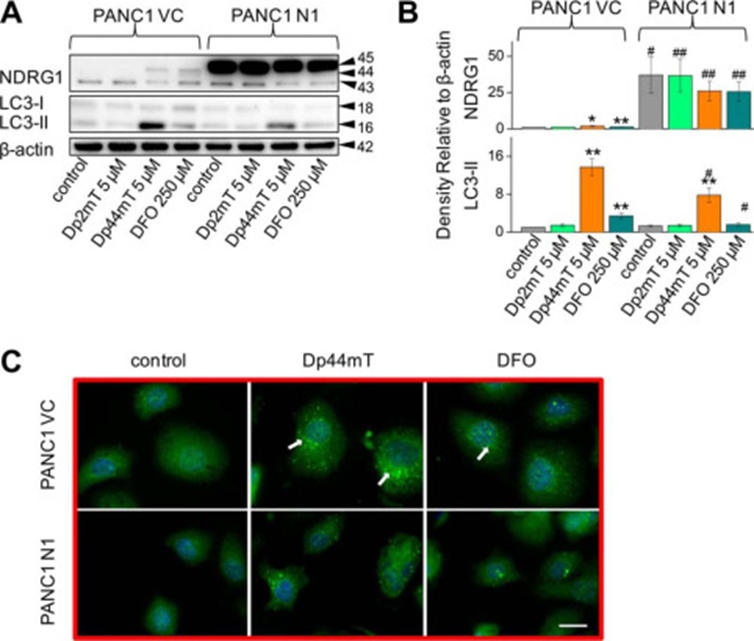
Figure 2: Iron chelation by DFO increases LC3-II levels and LC3-II-containing autophagosome formation
Iron chelation by DFO increases LC3-II levels and LC3-II-containing autophagosome formation. A, PANC1 VC and N1 cells were incubated for 24 h at 37 °C with, Dp44mT (5 μm), or DFO (250 μm), and Western blotting was performed to examine their effect on NDRG1, LC3-I, or LC3-II levels. B, densitometric analysis (arbitrary units) of blots in A. C, immunofluorescence studies with anti-LC3 antibody using PANC1 VC and N1 cells after a 24-h, 37 °C incubation with Dp44mT (5 μm) or DFO (250 μm). Scale, 20 μm. The Western analysis and immunofluorescence images shown are typical of three experiments. Densitometric analysis is the mean ± S.E. (three experiments) normalized to β-actin: *, p < 0.05; **, p < 0.01 versus their respective controls. #, p < 0.05; ##, p < 0.01 versus their corresponding treatments in PANC1 VC cells.
In the landscape of medical treatments for iron overload, deferoxamine mesylate stands out not just as a drug but as a beacon of hope for patients grappling with the life-threatening consequences of iron toxicity. Its development and application have paved the way for significant advancements in patient care, highlighting the importance of targeted therapies in chronic conditions. As research continues to evolve, the legacy of deferoxamine mesylate serves as a testament to the relentless pursuit of medical innovation. The drug's enduring significance in treating iron overload conditions underscores the critical balance between efficacy and patient quality of life, a balance that will continue to guide future therapeutic developments. In the grand tapestry of medical achievements, deferoxamine mesylate is a vivid thread, weaving together decades of research, patient stories, and the ongoing quest for better, more accessible treatments.












Comments