Risuteganib (trifluoroacetate salt) (Synonyms: ALG-1001) |
| رقم الكتالوجGC48401 |
An anti-integrin peptide
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
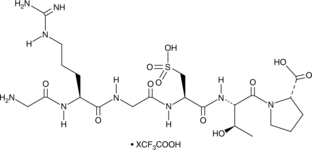
Risuteganib is an anti-integrin peptide and a derivative of RGD peptide .1 Risuteganib (400 µM) protects against hydroquinone-induced necrosis and apoptosis, as well as inhibits hydroquinone-induced production of reactive oxygen species (ROS), in human retinal pigment epithelial (RPE) cells.2 It decreases the expression of BAX, VEGFA, and ITB1 in human age-related macular degeneration (AMD) cybrid cell lines when used at a concentration of 20 mg/ml.3 Risuteganib arrests aberrant blood vessel growth mediated by αVβ3, αVβ5, and α5β1 integrins in vivo.1
1.Kaiser, P., Boyer, D.D., Campochiaro, P.A., et al.Integrin peptide therapy: The first wet AMD experienceInvest. Opth. Vis. Sci.54(15)2177(2013) 2.Yang, P., Shao, Z., Besley, N.A., et al.Risuteganib protects against hydroquinone-induced injury in human RPE cellsInvest. Ophthalmol. Vis. Sci.61(10)35(2020) 3.Differential effects of risuteganib and bevacizumab on AMD cybrid cellsExp. Eye Res.203108287(2021)
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















