Contezolid acefosamil sodium (Synonyms: MRX-4 sodium) |
| رقم الكتالوجGC62902 |
Contezolid acefosamil sodium (MRX-4) ، وهو أوكسازوليدينون الجديد والفعال عن طريق الفم ، هو مضاد حيوي قيد الدراسة لعلاج التهابات الجلد والأنسجة الرخوة المعقدة (cSSTI) التي تسببها البكتيريا المقاومة إيجابية الجرام
Products are for research use only. Not for human use. We do not sell to patients.
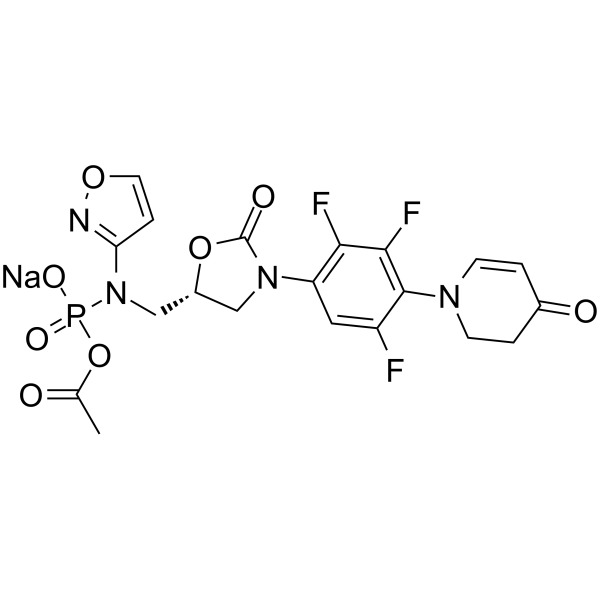
Cas No.: 1807365-35-0
Sample solution is provided at 25 µL, 10mM.
Contezolid acefosamil sodium (MRX-4), a new and orally active oxazolidinone, is an antibiotic in study for complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria. Contezolid acefosamil sodium (MRX-4) markedly reduces potential for myelosuppression and monoamine oxidase inhibition (MAOI)[1][2].
Contezolid (MRX-I) is highly potent against all Grampositive clinical isolates of staphylococci, streptococci, and enterococci, including MDR organisms such as MRSA, methicilline-resistant Streptococcus epidermidis (MRSE), penicillin-resistant Streptococci (PRSP), and VRE[2].
Oral absorption of Contezolid (MRX-I) occurrs rapidly in mouse, rat, and dog, with peak plasma concentrations observed at 0.5-2.6 h postdose. In mouse, rat, and dog, respectively, PK parameters are determined as follows: dose-normalized Cmax/dose was 524, 1065, and 259 ng/mL/(mg/kg); dose-normalized AUC0-t/dose was 1654, 3703, and 1664 ng•h/mL/(mg/kg); T1/2 is 1, 1.5, and 3 h; and the oral bioavailability is 69%, 109%, and 37%[2].Contezolid (MRX-I) exhibits no obvious toxicity[2].Contezolid (MRX-I, 100 mg/kg, once daily) significantly reduced the bacterial load in lungs compared to the untreated early and late controls[3].
[1]. Junzhen Wu, et al. Evaluation of the Effect of Contezolid (MRX-I) on the Corrected QT Interval in a Randomized, Double-Blind, Placebo- and Positive-Controlled Crossover Study in Healthy Chinese Volunteers. Antimicrob Agents Chemother. 2020 May 21;64(6):e02158-19.
[2]. Mikhail F Gordeev, et al. New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J Med Chem. 2014 Jun 12;57(11):4487-97.
[3]. Carolyn Shoen, et al. In Vitro and In Vivo Activities of Contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00493-18.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















