2-Methoxycinnamic acid |
| رقم الكتالوجGC35092 |
2-حمض الميثوكسيناميك هو مثبط غير تنافسي لتيروزيناز
Products are for research use only. Not for human use. We do not sell to patients.
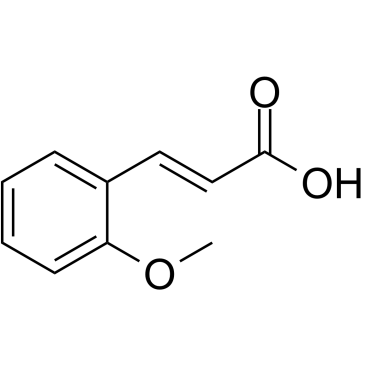
Cas No.: 6099/3/2
Sample solution is provided at 25 µL, 10mM.
2-Methoxycinnamic acid is a noncompetitive inhibitor of tyrosinase[1].
[1]. Lee HS, et al. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem. 2002 Mar 13;50(6):1400-3.
Review for 2-Methoxycinnamic acid
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
Review for 2-Methoxycinnamic acid
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















