Manitimus (FK778) (Synonyms: FK778) |
| رقم الكتالوجGC31810 |
مانيتيموس (FK778) هو مثبط لنزعة هيدروجيناز ديهيدروتورات ، وعقار فعال مثبط للمناعة.
Products are for research use only. Not for human use. We do not sell to patients.
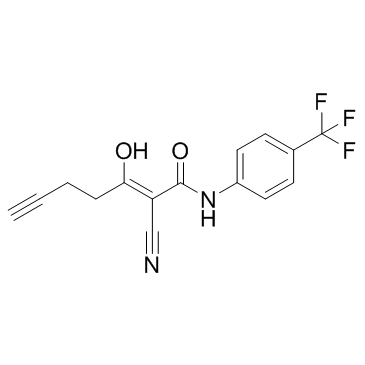
Cas No.: 202057-76-9
Sample solution is provided at 25 µL, 10mM.
Manitimus is an inhibitor of dehydroorotate dehydrogenase, and a potent immunosuppressive drug.
In the Manitimus-treated rats, there is a dose-related, differential effect: mean survival is 15.7 days in group 4 (Manitimus 5 mg/kg), 19.1 days in group 5 (Manitimus 10 mg/kg) and 25.4 days in group 6 (Manitimus 20 mg/kg)[1]. Manitimus (15 mg/kg, p.o.) results in a significant decrease in neointimal area and percentage of stenosis versus the control rats, and diminishes the effect that CMV infection results in a significant increase in intimal and medial cross-sectional area and medial wall thickness of the vein grafts[2].
[1]. Birnbaum F, et al. The new malononitrilamide immunosuppressant FK778 prolongs corneal allograft survival in the rat keratoplasty model. Eye (Lond). 2007 Dec;21(12):1516-23. Epub 2007 Mar 30. [2]. Kloppenburg G, et al. FK778 attenuates cytomegalovirus-enhanced vein graft intimal hyperplasia in a rat model. Intervirology. 2009;52(4):189-95.
Average Rating: 5 (Based on Reviews and 39 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















